Advances of surface-enhanced Raman and IR spectroscopies
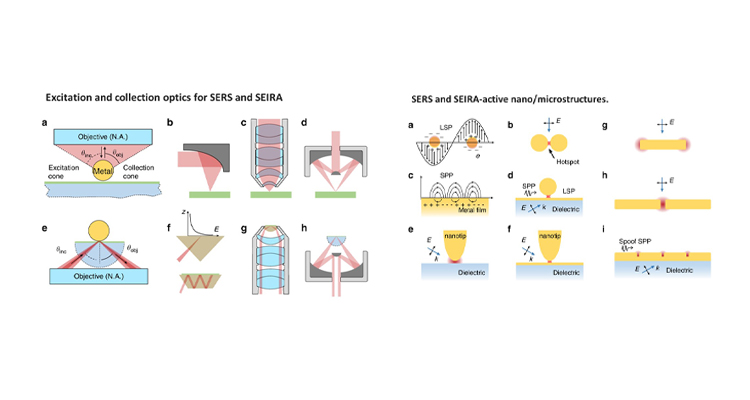 Dr Rajapandiyan Paneerselvam from the Department of Chemistry has published a paper titled “Advances of surface-enhanced Raman and IR spectroscopies: from nano/microstructures to macro-optical design” in the journal Light: Science & Applications, Volume 10, Article number: 161 (2021) having an Impact factor of 17.7.
Dr Rajapandiyan Paneerselvam from the Department of Chemistry has published a paper titled “Advances of surface-enhanced Raman and IR spectroscopies: from nano/microstructures to macro-optical design” in the journal Light: Science & Applications, Volume 10, Article number: 161 (2021) having an Impact factor of 17.7.
Raman and infrared (IR) spectroscopy are powerful analytical techniques, which are widely used for a variety of applications including food analysis, environmental analysis, chemical, and biomolecule analysis. This review article presents some latest advancements in vibrational spectroscopic techniques, and further developments in this field are given with emphasis on emerging techniques and methodologies.
This article has been published with Prof Zhong-Qun Tian’s group, State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China.
Furthermore, Dr Rajapandiyan’s research group will focus on the development of plasmonic nanostructures for surface-enhanced Raman spectroscopy and its applications in food science, spectroelectrochemistry, and microfluidics in the future.
Read the full paper here: https://doi.org/10.1038/s41377-021-00599-2
- Published in Chemistry-news, Departmental News, News, Research News
Prof U Ramamurty, renowned researcher from NTU Singapore, visits SRM University-AP
 An interactive session between Prof U Ramamurty, President Chair Professor, School of Mechanical & Aerospace Engineering at Nanyang Technological University (NTU), Singapore, and the faculty members of SRM University – AP, Andhra-Pradesh was held on Monday.
An interactive session between Prof U Ramamurty, President Chair Professor, School of Mechanical & Aerospace Engineering at Nanyang Technological University (NTU), Singapore, and the faculty members of SRM University – AP, Andhra-Pradesh was held on Monday.
During the discussion, Prof Ramamurty emphasized the importance of research collaboration between faculty members from different research areas and about utilizing expertise to achieve significant scientific output.
Dr Pardhasaradhi Maram from the Department of Chemistry, Dr Sabyasachi Mukhopadhyay from the Department of Physics, and Prof G S Vinod Kumar from the Department of Mechanical Engineering presented their detailed research areas that focus on storage devices, catalysts for value-added products, energy and sensing devices, novel metallic materials, additive manufacturing of metals and Bio-implants, and industry collaborative research work.
Prof Ramamurty said that he is glad to see that productive science is being done at SRM University-AP. “Given that the University has started only 4 years ago and been functioning amidst a pandemic for more than one and a half years, the progress in research is significant and very impressive. Interdisciplinary efforts between various departments in the University will give effective results”, he added.
Prof D Narayana Rao, Pro-Vice-Chancellor, SRM University – AP expressed his interest in establishing NTU – SRM joint Centre for Advanced Research in functional and structural materials at SRM University campus to Prof Ramamurty. The centre that Prof Rao envisions will provide an opportunity to synergize the expertise and resources of NTU, Singapore, and SRM University – AP to carry out front-line research in the areas of novel materials, self-healing materials and also additive manufacturing (3D Printing of metals and bio-implants).
- Published in Chemistry-news, Departmental News, Mechanical Engineering NEWS, News, Physics News, Research News
Simple and portable spectrochemical probe for rapid detection of chlorides ions in water
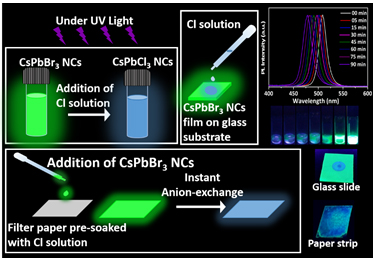 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Ms V.G.Vasavi Dutt and Mr Syed Akhil has published a research article titled Cesium Lead Bromide Perovskite Nanocrystals as a Simple and Portable Spectrochemical Probe for Rapid Detection of Chlorides in the Journal ChemistrySelect (Publisher: Wiley-VCH on behalf of Chemistry Europe, Impact Factor-2.2).
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Ms V.G.Vasavi Dutt and Mr Syed Akhil has published a research article titled Cesium Lead Bromide Perovskite Nanocrystals as a Simple and Portable Spectrochemical Probe for Rapid Detection of Chlorides in the Journal ChemistrySelect (Publisher: Wiley-VCH on behalf of Chemistry Europe, Impact Factor-2.2).
Chloride anions are widely abundant in water and when they combine with calcium, potassium, and magnesium, they form chloride salts. However, the higher concentrations badly affect the environment by causing severe dehydration and even plant death. High concentrations of sodium chloride exhibit the potential of corrosive damage thereby releasing toxic metals from plumbing fixtures. Hence, there is a need to monitor the concentration levels of chloride salts in water. Several techniques like titration, spectrophotometry, ion chromatography, electrochemistry, etc have been reported to date. Despite the high accuracy and precision of these techniques, they involve expensive instrumentation and is out of reach from on-site detection. Hence, it is necessary to look for simple, portable, and cost-effective strategies for the detection of chlorides in the water.
In this article, Dr Mishra’s research group demonstrated that the wide spectral tunability of CsPbBr3 perovskite nanocrystals (NCs) via instantaneous and facile anion exchange, make them a suitable candidate for chloride detection. Rapid anion-exchange processes between CsPbBr3 perovskite NCs and different chloride solutions were carried out in ambient conditions. The resultant anion-exchanged CsPbCl3-xBrx NCs preserved the structural properties and exhibited a remarkable blue shift in photoluminescence spectra. This forms a basis for the detection of chloride ions in water. This has been applied with the limit of detection up to 100 µM. The detection strategies were not only limited to the direct addition of chloride solutions to NCs, but they also showed a visual colour change under UV light when the chloride solution is drop-casted on CsPbBr3 films that are deposited on glass substrates. Furthermore, the detection strategy is established by drop-casting CsPbBr3 NCs onto paper strips that are pre-soaked in chloride solutions. A considerable blue shift in fluorimetry proves them to be an excellent sensing medium as practical spectrochemical probes for on-site detection of chlorides. Based on this, a colour chart and selectivity chart to access the presence of chlorides and their concentration is also demonstrated.
- Published in Chemistry-news, Departmental News, News, Research News
Third year CSE students innovate efficient plastic recycling technology
 Swikriti Khadke, Pragya Gupta, and Shanmukh Rachakunta from third-year Computer Science Engineering have published a research paper titled “Efficient Plastic Recycling and Remold Circular Economy using the Technology of Trust – Blockchain” along with their mentors from SRM University-AP Dr Jatindra Kumar Dash, Dr Goutam Kumar Dalapati and Dr Sabyasachi Chakrabortty in the peer-reviewed journal Sustainability.
Swikriti Khadke, Pragya Gupta, and Shanmukh Rachakunta from third-year Computer Science Engineering have published a research paper titled “Efficient Plastic Recycling and Remold Circular Economy using the Technology of Trust – Blockchain” along with their mentors from SRM University-AP Dr Jatindra Kumar Dash, Dr Goutam Kumar Dalapati and Dr Sabyasachi Chakrabortty in the peer-reviewed journal Sustainability.
Global plastic waste is increasing rapidly. The strategic management of plastic waste and recycling can preserve environmental species and associated costs. The utilization of plastic can be done by introducing Blockchain during plastic waste recycling. Automation for the segregation and collection of plastic waste can effectively establish a globally recognizable tool using Blockchain-based applications. Collection and sorting of plastic recycling are feasible by keeping track of plastic with unique codes or digital badges throughout the supply chain. Efficient recycling technology is essential to reduce plastic pollution. Many technologies have been employed to enhance plastic recycling. Among them, blockchain is promising for plastic recycling and circular economy (CE). Blockchain, a distributed ledger, consists of some ordered blocks which are unchangeable. This can be considered an exemplary way to push the transactions of their customers under the same blockchain technology. The research group used machine learning techniques to predict plastic generation globally so that they could see the impact it will make in the coming future. The students have used ARIMA – Auto-Regressive Integrated Moving Average for the study.
The potential idea is to utilize an approach wherein recyclers can keep track of generated waste as it moves through the various chains. A platform that works by tracking recycling activities across a local recycling supply chain on the Blockchain. When this will be publicly available, consumers can also use the ledger info to make more informed purchasing decisions. The Blockchain can be utilized to track individual items through the recycling supply chain by creating physical markers like QR codes.
The suggested Blockchain-based platform can be implemented in various nations with an autonomous waste collector and storage system. This process can be expanded to individual collectors and storage systems. The novel process will be created by incorporating a reward-based Blockchain scheme with the collaboration of global businesses and local waste collectors. The proposed model further allows the effective sharing of databases among various supply chains to create a CE.
Talking about the social implications of the research, the students firmly believe that the study will result in the introduction of new technology in the recycling industry and promote awareness about technology in rural areas. Developing a platform and implementing blockchain and other facilities will be the focus of these young innovative brains of SRM University-AP in the forthcoming days.
Read the full paper here: https://doi.org/10.3390/su13169142
- Published in Chemistry-news, Computer Science News, Departmental News, News, Physics News, Research News
Impact of Surface Chemistry on the Excited State Interactions of CsPbBr3
 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP along with his team comprising of his PhD scholars Mr. Syed Akhil, Ms. V.G.Vasavi Dutt, and Mr Rahul Singh have published a research article titled “Surface-State-Mediated Interfacial Hole Transfer Dynamics Between CsPbBr3 Perovskite Nanocrystals and Phenothiazine Redox Couple” in The Journal of Physical Chemistry-C, published by The American Chemical Society with an impact factor of ~4.126.
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP along with his team comprising of his PhD scholars Mr. Syed Akhil, Ms. V.G.Vasavi Dutt, and Mr Rahul Singh have published a research article titled “Surface-State-Mediated Interfacial Hole Transfer Dynamics Between CsPbBr3 Perovskite Nanocrystals and Phenothiazine Redox Couple” in The Journal of Physical Chemistry-C, published by The American Chemical Society with an impact factor of ~4.126.
Dr Mishra’s research interests lie in Semiconductor nanocrystals, Core/shell branched structures, Nanowires, Perovskite nanocrystals and Optoelectronic device fabrication. He studied the role of surface chemistry for improving excited state hole transfer from CsPbBr3 nanocrystals to an acceptor, potentially applicable for photocatalytic applications.
About the research:
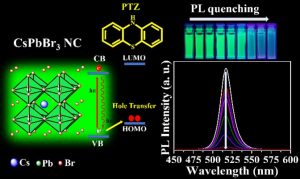 Recently, caesium lead bromide (CsPbBr3) perovskite nanocrystals (PNCs) gained enormous attention for designing photocatalytic reactions because of their photocatalytic properties. But the surface chemistry of nanocrystals is often ignored which dictate the excited state interactions of these semiconductor nanocrystals with the charge shuttling redox-active molecules. In this work, we have explored the impact of CsPbBr3 perovskite nanocrystals with the three different surface chemistries on the excited state interactions with the standard hole acceptor phenothiazine molecule. From the steady PL-lifetime decay measurements we have calculated the photoinduced hole transfer (PHT). In the amine-free PNCs case, PHT is 6 times higher than the conventional amine capped ligands. Using the lifetime fast component (1) rate constants, we have calculated the hole transfer constant (kht) which is 3.942 × 108 s-1 and it is 4 times higher in amine-free ligands when compared with conventional amine ligands system.
Recently, caesium lead bromide (CsPbBr3) perovskite nanocrystals (PNCs) gained enormous attention for designing photocatalytic reactions because of their photocatalytic properties. But the surface chemistry of nanocrystals is often ignored which dictate the excited state interactions of these semiconductor nanocrystals with the charge shuttling redox-active molecules. In this work, we have explored the impact of CsPbBr3 perovskite nanocrystals with the three different surface chemistries on the excited state interactions with the standard hole acceptor phenothiazine molecule. From the steady PL-lifetime decay measurements we have calculated the photoinduced hole transfer (PHT). In the amine-free PNCs case, PHT is 6 times higher than the conventional amine capped ligands. Using the lifetime fast component (1) rate constants, we have calculated the hole transfer constant (kht) which is 3.942 × 108 s-1 and it is 4 times higher in amine-free ligands when compared with conventional amine ligands system.
According to Dr Nimai Mishra, the most important contribution of this research is that these results highlight the impact of surface chemistry on the excited state interactions of CsPbBr3 PNCs and conclude amine-free PNCs could be an ideal candidate for photocatalytic reactions.
Read the full paper: https://pubs.acs.org/doi/10.1021/acs.jpcc.1c07129
- Published in Chemistry-news, News, Research News
Dr Satheesh Ellipilli joins us as a Ramanujan Fellowship Faculty
 SRM University-AP is honoured to host Dr Satheesh Ellipilli as a DST- Ramanujan Fellowship Faculty and facilitate his research for the next five years.
SRM University-AP is honoured to host Dr Satheesh Ellipilli as a DST- Ramanujan Fellowship Faculty and facilitate his research for the next five years.
Ramanujan Fellowship is one of the most prestigious scientific fellowships that is offered to the Indian scientists working abroad. This fellowship is offered by Science and Engineering Research Board (SERB) to encourage scientists of Indian origin to return and research in an Indian institute/University.
SERB offers the scientists Rs 1,35,000/- per month along with research grant of Rs 7,00,000/- per annum and Rs 60,000/- per annum for overhead charges.
Dr. Satheesh Ellipilli obtained his PhD from Indian Institute of Science Education and Research, Pune. He worked as a postdoctoral researcher in The Ohio State University (Columbus, USA), Emory University (Atlanta, USA), and The University of Utah (Salt Lake City, USA).
Dr. Satheesh Ellipilli has extensive experience in the field of nucleic acid chemistry, particularly, focusing on utilization of RNA nanotechnology for cancer therapy using RNAi therapeutics in combination with small molecule drugs.
He has made numerous publications in some of the most renowned journals like Journal of Organic Chemistry, Journal of Controlled Release, Chemical Communications, Bioconjugate Chemistry, Organic and Biomolecular Chemistry, and Chemical Review to name a few.
Having Dr Ellipilli with us for the duration of his fellowship is a golden learning opportunity and a pleasure to be the host institution for his work. We hope that our students and scholars develop stronger research ethics and acumen in his company.
- Published in Chemistry-news, Departmental News, News
Top 5% most cited author: Royal Society of Chemistry
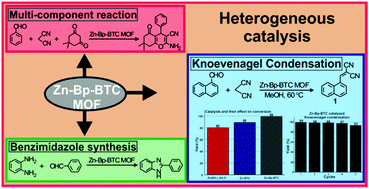 SRM University-AP could not be more proud to announce that Dr S Mannathan, Head of Department of Chemistry has made it to the top 5% in the list of the Most Cited Authors by the Royal Society of Chemistry. It is inspiring to have a faculty member in our midst whose work has helped and facilitated the research of so many others.
SRM University-AP could not be more proud to announce that Dr S Mannathan, Head of Department of Chemistry has made it to the top 5% in the list of the Most Cited Authors by the Royal Society of Chemistry. It is inspiring to have a faculty member in our midst whose work has helped and facilitated the research of so many others.
Dr Mannathan obtained his doctorate from National Tsing Hua University, Taiwan. His research interests primarily lie in Metal-catalyzed organic transformation reactions, Multicomponent reactions, and Asymmetric synthesis. His research followed by scientists all over the world leading him to become one of the top 5% authors in terms of citations
In the field of Transition Metal Complexes as Catalysts in Organic Reactions, he particularly leans towards ‘Nickel-and cobalt-catalyzed three-component coupling and reductive coupling reactions’, and ‘Palladium-catalyzed reductive arylation’. Similarly, in Asymmetric Synthesis, he favours research into ‘Asymmetric reductive Heck reaction for the synthesis of chiral indanones’, and ‘Synthesis of bicyclic tertiary alcohols and its related asymmetric version via reductive [3+2] cycloaddition reaction by using chiral cobalt complexes.’
About the top 5% most cited paper:
In this work, he reported the synthesis and application of a Zn-Bp-BTC MOF (Bp – 4,4′-bipyridine; BTC – 1,3,5-benzene tricarboxylic acid; MOF – metal organic framework) as a heterogeneous catalyst for mediating organic reactions. Initial reaction conditions were optimized for the Knoevenagel condensation reaction using Zn-Bp-BTC as a heterogeneous catalyst. Various factors such as the effect of solvent, temperature and catalyst loading were evaluated. Although the reaction proceeded at room temperature using methanol as the solvent, 60 °C offered the best yield in a shorter duration. Under optimized reaction conditions, a wide range of α,β-unsaturated dicyano compounds were prepared from the corresponding carbonyl precursor and malononitrile, the active methylene counterpart. A systematic investigation was also carried out to assess the role of the ligand and metal salt in the Knoevenagel condensation reaction. It was found that the Zn-Bp-BTC MOF catalyzed the reaction efficiently in comparison to its analogue Zn-BTC MOF and precursor Zn(NO 3 ) 2 ·6H 2 O. Finally, catalytic recycling and stability studies showed that the catalyst is able to mediate the reaction for up to five consecutive cycles without undergoing any significant chemical or morphological changes. Further, the catalyst was tested for its efficacy in a multicomponent reaction (MCR). An MCR with the Zn-Bp-BTC MOF as the catalyst afforded good yields and there was no reaction in the absence of the catalyst. Similarly, the catalyst was tested for its efficiency in benzimidazole synthesis.
Dr Mannathan did this research in collaboration with Dr. Kathiresan Murugavel, Scientist, Electro Organic Division, CSIR-Central Electrochemical Research Institute (Govt of India), Karaikudi.
- Published in Chemistry-news, Departmental News, News, Research News
Dr Nimai Mishra on Impact of shell thickness on photostability studies of green-emitting “Giant” quantum dots
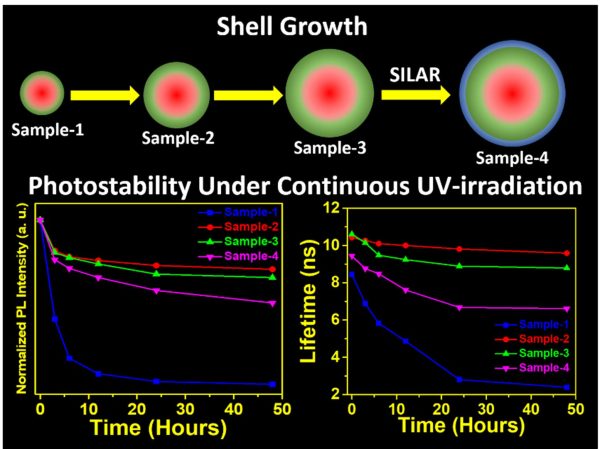 SRM University-AP is pleased to announce that Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Mr Rahul Singh, Mr Syed Akhil, and Ms V.G.Vasavi Dutt, has published a research article titled “Shell thickness-dependent photostability studies of green-emitting “Giant” quantum dots” in the journal Nanoscale Advances (The Royal Society of Chemistry) with an impact factor of ~4.533.
SRM University-AP is pleased to announce that Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Mr Rahul Singh, Mr Syed Akhil, and Ms V.G.Vasavi Dutt, has published a research article titled “Shell thickness-dependent photostability studies of green-emitting “Giant” quantum dots” in the journal Nanoscale Advances (The Royal Society of Chemistry) with an impact factor of ~4.533.
About the research:
Highly efficient green-emitting core/shell giant quantum dots have been synthesized through a facile “one-pot” gradient alloy approach. Furthermore, an additional ZnS shell was grown using the “Successive Ionic Layer Adsorption and Reaction” (SILAR) method. Due to the faster reactivity of Cd and Se compared to an analogue of Zn and S precursors it is presumed that CdSe nuclei are initially formed as core and gradient alloy shells simultaneously encapsulate the core in an energy-gradient manner and eventually thick ZnS shells were formed. Using this gradient alloy approach, we have synthesized four different sized green-emitting giant core-shell quantum dots to study their shell thickness-dependent photostability under continuous UV irradiation, and temperature-dependent PL properties of nanocrystals. There was a minimum effect of the UV light exposure on the photostability after a certain thickness of the shell. The QDs diameter of ≥ 8.5 nm shows substantial improvement in photostability compared to QDs with a diameter ≤ 7.12 nm when continuously irradiated under the strong UV light (8 W/cm2, 365 nm) for 48 h. The effect of temperature on the photoluminescence intensities was studied with respect to shell thickness. There were no apparent changes in PL intensities observed for the QDs ≥ 8.5 nm, on the contrary, for example, QDs with < 8.5 nm in diameter (for ~7.12 nm) show a decrease in PL intensity at higher temperatures ̴90°C.
More importantly, these results highlight the synthesized green-emitting gradient alloy QDs with superior optical properties can be used for highly efficient green emitters and are potentially applicable for the fabrication of green LEDs.
Read the full paper: https://pubs.rsc.org/en/content/articlelanding/2021/NA/D1NA00663K
- Published in Chemistry-news, Departmental News, News, Research News
Dr Nimai Mishra on The Impact of Shell Thickness on Charge Transfer Dynamics in Green Emitting Core/Shell Giant Quantum Dots
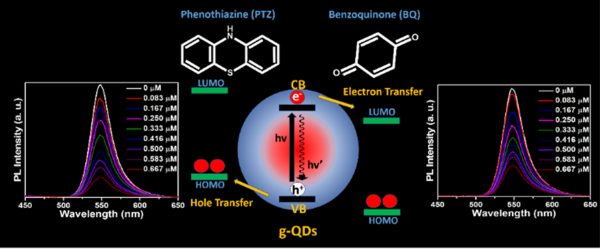 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Mr Rahul Singh, Mr Syed Akhil, and Ms V. G. Vasavi Dutt, have published a research article titled “Study of Shell Thickness Dependent Charge Transfer Dynamics in Green Emitting Core/Shell Giant Quantum Dots” Nature Index journal, “Inorganic Chemistry” published by The American Chemical Society having an impact factor of 5.1.
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Mr Rahul Singh, Mr Syed Akhil, and Ms V. G. Vasavi Dutt, have published a research article titled “Study of Shell Thickness Dependent Charge Transfer Dynamics in Green Emitting Core/Shell Giant Quantum Dots” Nature Index journal, “Inorganic Chemistry” published by The American Chemical Society having an impact factor of 5.1.
About the paper:
The superior photostability enables green-emitting graded alloy core/shell giant quantum dots (g-QDs) for optoelectronic application. However, it is essential to understand how the shell thickness affects interfacial charge separation. This work explores the impact of shell thickness on photoinduced electron transfer (PET) and photoinduced hole transfer (PHT) with an electron acceptor benzoquinone and a hole acceptor phenothiazine, respectively. The four graded alloy core/shell green-emitting g-QDs with different shell thicknesses were synthesised. The PET and PHT rate constants were obtained from photoluminescence and PL-lifetime decay measurement. Our study concludes that g-QDs with a diameter ~7.14 show a substantial improvement in charge transfer than g-QDs ≥ 8.5 nm in diameter. Similarly, the PET and PHT rates are 3.7 and 4.1 times higher for 7.14 nm g-QDs than for the 10.72 nm sample. The calculated electron and hole transfer rate constant (ket/ht) of g-QD with 7.14 nm in diameter are 10.80 × 107 s-1 and 14 × 107 s-1, which shows 8.5 and 8 times higher compared to g-QDs with a 10.72 nm in diameter.
Industrial implications:
More importantly, these results highlight the impact of shell thickness on the excited state interactions of green-emitting g-QDs and conclude that g-QDs with a relatively thin shell can be a better choice as photoactive materials for photocatalysts, photodetectors, and solar cells.
Want the complete details of Dr Nimai Mishra’s paper?
- Published in Chemistry-news, Departmental News, News
High-quality perovskite nanocrystals for light-emitting applications
 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under his supervision, Ms VG Vasavi Dutt, Mr Syed Akhil, Mr Rahul Singh, and Mr Manoj Paalabathuni have published a research article titled “High-Quality CsPbX3 (X = Cl, Br, or I) Perovskite Nanocrystals Using Ascorbic Acid Post-Treatment: Implications for Light-Emitting Applications” in the Journal “ACS Applied Nano Materials” (published by The American Chemical Society) having an impact factor of ~5.1.
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under his supervision, Ms VG Vasavi Dutt, Mr Syed Akhil, Mr Rahul Singh, and Mr Manoj Paalabathuni have published a research article titled “High-Quality CsPbX3 (X = Cl, Br, or I) Perovskite Nanocrystals Using Ascorbic Acid Post-Treatment: Implications for Light-Emitting Applications” in the Journal “ACS Applied Nano Materials” (published by The American Chemical Society) having an impact factor of ~5.1.
Abstract:
Cesium lead halide perovskite nanocrystals (CsPbX3 PNCs) have been the flourishing area of research in the field of photovoltaic and optoelectronic applications because of their excellent optical and electronic properties. However, they suffer from low stability and deterioration of photoluminescence (PL) properties post-synthesis. One of the ways to minimize the surface defects in the surface treatment with suitable ligands is to achieve the PNCs with superior PL properties for light-emitting applications.
In this article, Dr Mishra’s research group addressed the issue of stability in PNCs. We demonstrate to achieve high photoluminescence and stability of CsPbX3 PNCs by incorporating ascorbic acid via post-treatment as a new capping ligand that is abundantly available. Upon addition of ascorbic acid as surface passivation ligand into the oleic acid/oleylamine system to get near-unity photoluminescence quantum yield (PLQY) of CsPbBr3, CsPb(Br/I)3, and for CsPbI3 perovskite NCs. Maintaining stability has become the hotspot of research in this field. Hence, as-a-proof of concept, the stability studies of PNCs in ambient conditions, under continuous UV irradiation, and PL with temperature variations are put forth here. The stability enhancement with post-treatment of ascorbic acid is highly reproducible as we tested for four batches of samples.
Despite the significant advancements of PNCs, there is a challenge afflicting the stability of CsPbI3 PNCs. They are thermodynamically unstable and undergo a non-perovskite phase (δ-phase) transition at room temperature. Many efforts have been reported in the stabilization of iodide perovskite NCs by critically passivating PNCs and applying them for optoelectronics and photovoltaics. On the other hand, mixed halide perovskites like CsPbBrI2 which are relatively stable than CsPbI3 PNCs are a better choice for device applications. But, photo-induced halide segregation is unavoidable which in turn again limit their usage in practical applications. In this manuscript, we demonstrated that the ultra-stable iodide-based PNCs can be achieved by simple and facile surface treatment with ascorbic acid.
The PL intensity of untreated and ascorbic acid-treated PNCs is recorded for 42 days since the date of synthesis. The measurements are carried out for 4 different batches of samples to ensure reproducibility. It is found that the PL intensity is deteriorating rapidly for untreated PNCs while the PL intensity is largely maintained for ascorbic acid treated PNCs. Nearly ̴72% of the initial PL intensity is maintained even after 42 days for the ascorbic acid-treated CsPbBr3 PNCs while the PL intensity is dropped to 24% for untreated PNCs. Ascorbic acid treated CsPbBrI2 PNCs exhibited exceptional ambient stability where ̴69% of the initial PL intensity is maintained after 42 days while the PL of untreated CsPbBrI2 PNCs is degraded rapidly within 2 weeks from the date of synthesis. Moreover, the PL stability of CsPbI3 PNCs is high for ascorbic acid-treated samples even after 55 days while the PL has deteriorated within 4 days for untreated CsPbI3 PNCs. The PL of untreated CsPbI3 PNCs is completely lost in the first 4 hours of UV illumination while ̴ 76.7% remnant PL is observed for ascorbic acid-treated CsPbI3 PNCs. We believe the stabilization of CsPbX3 PNCs of different halide compositions via simple surface treatment with ascorbic acid could form a basis for futuristic light-emitting applications.
Read the full paper: https://pubs.acs.org/doi/full/10.1021/acsanm.1c04312
- Published in Chemistry-news, News, Research News











