 SRM Univeristy-AP is proud to announce that Prof. Ranjit Thapa, Department of Physics, has obtained a prestigious SERB-DST grant of Rs. 32 lakhs for a period of three years for his project, “Design Principle of Single Atom Catalyst for Nitrogen Fixation over HER: Energy Parameter, Electronic Descriptor and Database”.
SRM Univeristy-AP is proud to announce that Prof. Ranjit Thapa, Department of Physics, has obtained a prestigious SERB-DST grant of Rs. 32 lakhs for a period of three years for his project, “Design Principle of Single Atom Catalyst for Nitrogen Fixation over HER: Energy Parameter, Electronic Descriptor and Database”.
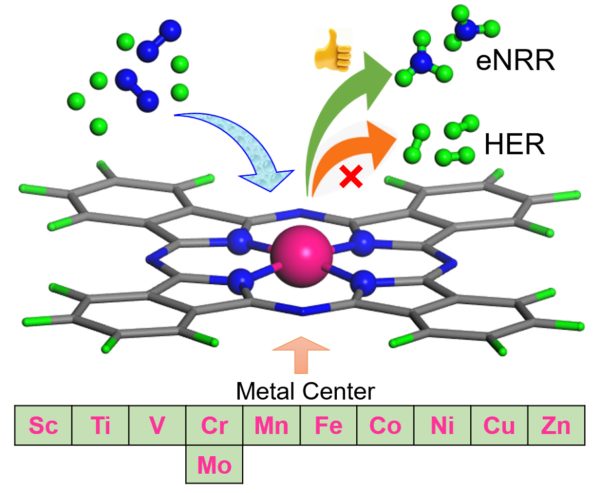
Ammonia (NH3) is the prime source of fertilizers and an important carrier of energy too. Ammonia can be stored in its chemical form for a long and it is easy to transport. So now researchers are looking forward to using ammonia in place of hydrogen as an energy source. But the production of ammonia with existing techniques needs more energy compared to the energy it stored in its chemical bond. So, an alternative process that is environmentally friendly and cost-effective is needed to be in place.
In 2019 the global production capacity of ammonia is 235 million metric tons and will increase to 290 million metric tons by 2030. The importance of ammonia is due to its application in broad and diverse fields, such as fertilizers, textiles, pharmaceuticals, and is a carbon-free energy carrier. The Haber-Bosch process is used for the synthesis of ammonia (NH3) from N2 and H2 using Fe based catalyst. But the process emits carbon dioxide (CO2) (1.5 tons of CO2/tons of NH3 production) requires high pressure and temperature and consumes around 2% of the global supply of energy. Electrocatalytic N2 fixation (N2 + 6H+ + 6e− → 2NH3) showed great potential due to the possible use of atmospheric nitrogen and hydrogen derived from water through electrolysis and in mild conditions. However, the slow kinetics of N2 adsorption, splitting of the strong N≡N bond are the challenges for the electrocatalytic NRR process. In the electrocatalytic NRR process, the fast reaction kinetics of hydrogen evolution reaction is the greatest obstacle. To solve these challenges, the search for various types of catalysts is on the roll.
To date, trial and error methods have been used to synthesize the catalysts for the electrocatalytic NRR process. Thanks to the rapid development of density functional theory-based computational methods, the intermediate steps during NRR can be identified at the atomic level, the underlying principles can be understood, and a large space of catalysts can be checked for efficient NRR within a limited time. Without understanding the correct electronic structure of SAC and its correlation with the overpotential of NRR and defining the correct energy parameter to define “NRR over HER” and “N2 binding over H binding free energy”, we can never design the best catalyst cost-effectively. We will address these problems through this project’s objectives.
The project will help to design the best single-atom catalyst for the reduction of nitrogen (from the air) through the electrocatalytic process and convert it into ammonia. The designed catalyst can be synthesis by the industry and can be used for NRR.
This project will help a step forward towards more ammonia production for the uses in the agriculture sector, energy sector, and related sector.

