The Department of Physics is glad to announce that Prof Ranjit Thapa and his PhD scholar, Mr Samadhan Kapse, have published a patent titled “Highly Stable Ruthenium Single-Atom Catalysts on Fe3O4/MWCNTs for Hydrogen Evolution Reaction” (Application no. 202241006087). The research was done in collaboration with Ms Shwetha K R, Mr Shivanna M and Dr Nagaraju D H, from the Department of Chemistry, School of Applied Sciences, REVA University, Bangalore.
A Brief Description of the Research
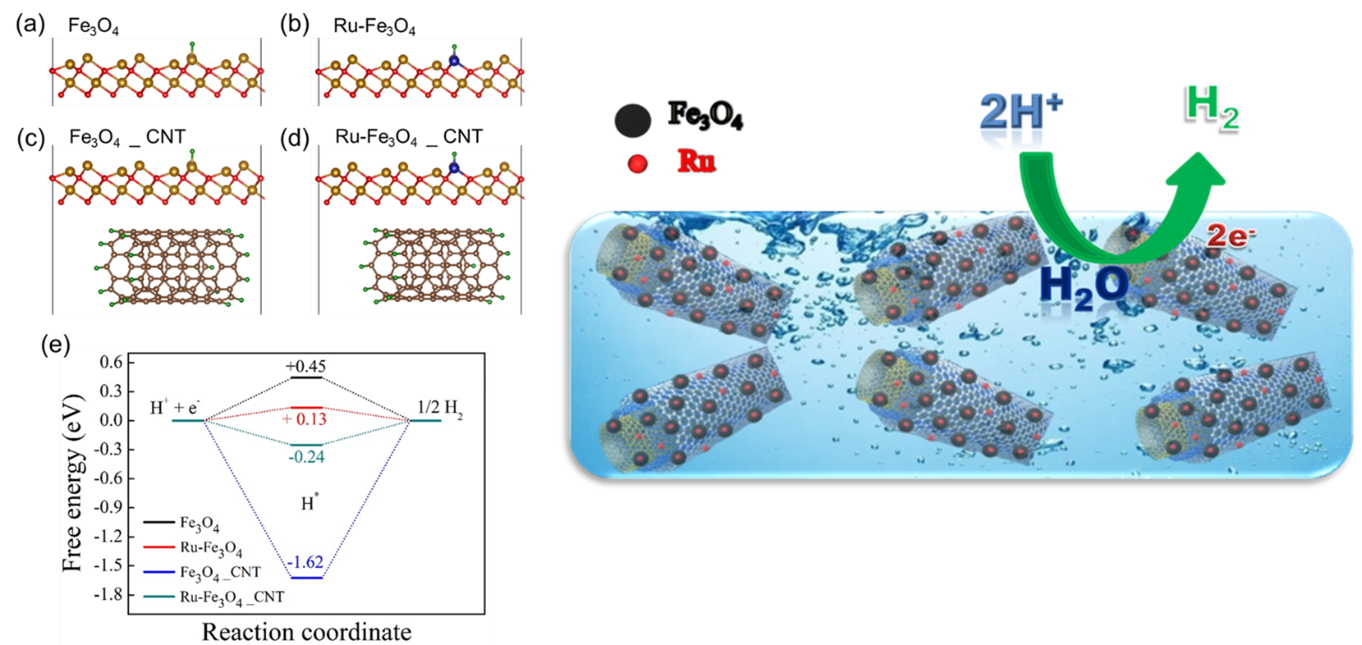
In the current work, Fe3O4 nanoparticles were prepared by a simple chemical co-precipitation method under an inert atmosphere, and it was utilised for HER studies. Ru nanoparticles were profitably deposited over Fe3O4/MWCNTs modified glassy carbon electrode by the electrochemical deposition technique. The superior HER activity was achieved on Fe3O4/MWCNTs/Ru in 0.1M H2SO4 aqueous media. We demonstrated that synthesised electrocatalyst offers low over potential 101 mV to reach a current density of 10 mA cm-2 towards hydrogen evolution reaction. It displays exceptional stability and finds to be of no change in the HER activity despite 1000 cycles. It is emphasised that a small weight percentage of ruthenium in the prepared catalyst can replace high-cost platinum in renewable energy technologies.
Social Implications of the Research
Production of renewable energy has greater significance in the present situation owing to the impact of the depletion of non-renewable energy resources such as fossil fuels and the release of greenhouse gases into the atmosphere. Hydrogen has gained considerable interest as an energy storage and energy carrier due to its high energy density (146kJ/g), and its utilisation also eliminates pollution and toxicity. Several methods have been explored to produce molecular hydrogen. Among them, the electrolysis of water is the best way to produce high purity hydrogen from water. An excellent electrocatalyst is obligatory to liberate hydrogen gas effectively from water. It is known that Superior HER activity has been achieved using platinum (Pt) and Pt-based catalysts. Due to its high cost and low surplus, its expansion has been limited to the industrial scale. The research proposes that Ru-based catalysts can overcome these challenges.
DFT study is more effective to find the origin of catalytic activity in materials for designing highly promising catalysts for various catalytic reactions. The researchers expressed their gratitude to SRM University-AP for providing the required computational facility and support.

