
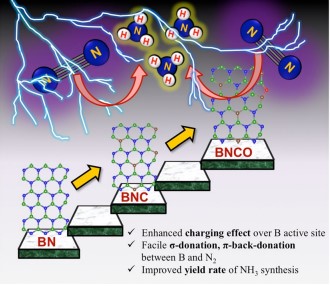
Significant measures have been undertaken to synthesise ammonia proficiently, the future renewable energy fuel for its wide range of applications in various sectors. On this account, a research paper titled “Oxygen functionalization-induced charging effect on boron active sites for high-yield electrochemical NH3 production” has been published by Prof. Ranjit Thapa, Professor, Department of Physics and his research scholar Mr Samadhan Kapse in the journal Nano-Micro Letters having an impact factor of 23.655.
Abstract
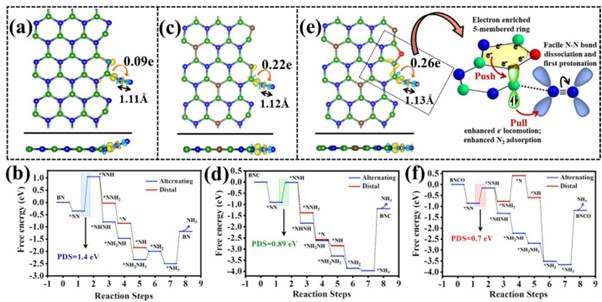
Ammonia has been recognized as the future renewable energy fuel because of its wide-ranging applications in H2 storage and transportation sector. In order to avoid the environmentally hazardous Haber–Bosch process, recently, the third-generation ambient ammonia synthesis has drawn phenomenal attention and thus tremendous efforts are devoted to developing efficient electrocatalysts that would circumvent the bottlenecks of the electrochemical nitrogen reduction reaction (NRR) like competitive hydrogen evolution reaction, poor selectivity of N2 on the catalyst surface. Herein, we report the synthesis of an oxygen-functionalised boron carbonitride matrix via a two-step pyrolysis technique. The conductive BNCO(1000) architecture, the compatibility of B-2pz orbital with the N-2pz orbital and the charging effect over B due to the C and O edge-atoms in a pentagon altogether facilitate N2 adsorption on the B edge-active sites. The optimum electrolyte acidity with 0.1 M HCl and the lowered anion crowding effect aid the protonation steps of NRR via an associative alternating pathway, which gives a sufficiently high yield of ammonia (211.5 μgh−1 mgcat−1) on the optimized BNCO(1000) catalyst with a Faradaic efficiency of 34.7% at −0.1 V vs RHE. This work thus offers a cost-effective electrode material and provides the contemporary idea about reinforcing the charging effect over the secured active sites for NRR by selectively choosing the electrolyte anions and functionalizing the active edges of the BNCO(1000) catalyst.
A brief summary of the research in layman’s terms
In summary, this work displayed the significant role of O and C doping within BN architecture to promote NRR on the edge B sites via an associative alternating mechanism. The gradual formation of the ideal structure was systematically studied by means of XPS and the electronic properties was investigated from NEXAFS. A greater impact was found on the charging effect of B centres due to O-functionalized edges that induced a greater charge density from B to the adsorbed N2, overcoming the potential determining steps for NRR. This work simultaneously highlighted the importance of the choice of electrolyte, where in 0.1 M HCl our catalyst BNCO(1000) yielded 211.5 μg h−1mgcat−1 of ammonia at −0.1 V vs RHE with a FE of 34.7%. It was experimentally found and theoretically supported that the bulky anions in H2SO4 and H3PO4 blocked the B active sites by a Lewis acid-base interaction between the B sites and the O ends of the anions, hence not suitable for this class of materials. Thus, the present work offered an overall idea of catalyst designing and the medium to retain a high and consistent NRR performance.
Social implications of the research
Nitrogen reduction reaction (NRR) performed electrochemically is regarded as a green and legitimate approach to ammonia synthesis and it has been intrinsically brought into the limelight by the worldwide research community, not only because of the immense use of ammonia in the agriculture and transportation sector but also due to urge to resolve the fallacies associated with the process. Primarily, the eternal problem persisting with NRR is the predominance of the combative hydrogen evolution reaction (HER) at the same potential range, which overpowers NRR over most of the catalyst surfaces, resulting in poor yield and Faradaic efficiency (FE) of ammonia production. Researchers thus majorly focus on varied catalyst development, which includes several strategies: (a) Selectivity of elements that would prefer binding with N2 over protons, (b) Blocking the HER active sites, (c) Phase-selective material designing, inhibiting HER at the active surface, (d) interface-engineering that would deviate the HER pathway inducing better Faradaic efficiency for NRR. Although either 1st-row transition metals or semimetals are regarded as more suitable candidates for NRR, a metal-free approach is rather preferred for the cost-effectiveness and simplicity of the process. Boron (B)-based electrocatalyst in this respect can act as a strong contender. The research also posits that Density functional theory is useful for the molecular level understanding to unveil the performance of different catalytic reactions.
Collaborations
- Ms Ashmita Biswas, Institute of Nano Science and Technology (INST), Sector-81, Mohali, Punjab 140306, India
- Mr Ramendra Sundar Dey, Institute of Nano Science and Technology (INST), Sector-81, Mohali, Punjab 140306, India

