 As technology gets revolutionized, we face the need to have more- more efficient energy storage solutions with decreased strain on the environment to meet our need. SRM University-AP happy to let everyone know that our Dr Ranjit Thapa, Professor of Physics along with his PhD scholar, Mr Deepak S Gavali has published a paper titled, “Understanding the role of lithium bonds in doped graphene nanoribbons as cathode hosts for Li-S batteries: A first-principles study” in ‘International Journal of Energy Research’, having an Impact Factor of 5.164.
As technology gets revolutionized, we face the need to have more- more efficient energy storage solutions with decreased strain on the environment to meet our need. SRM University-AP happy to let everyone know that our Dr Ranjit Thapa, Professor of Physics along with his PhD scholar, Mr Deepak S Gavali has published a paper titled, “Understanding the role of lithium bonds in doped graphene nanoribbons as cathode hosts for Li-S batteries: A first-principles study” in ‘International Journal of Energy Research’, having an Impact Factor of 5.164.
About the Research:
Lithium sulphur (Li-S) batteries hold immense potential as energy storage devices of the future because of their high energy density (2600 Wh kg −1), lower cost and non-toxic nature as compared to the currently available lithium-ion batteries. However, the commercialization of Li-S batteries is hindered due to a number of challenges that include the polysulfide shuttle effect, viz, the dissolution of lithium polysulfide species (LiPS) in the cathode into the electrolyte and its diffusion to the anode and back. The shuttle effect results in poor Coulombic efficiency, low utilization of active materials, and degradation of electrode. Moreover, the insulating nature of the sulphur cathode is a key contributor to its low specific capacity. One way to circumvent these problems is by employing conducting cathode hosts as additives that would act as trapping agents for the LiPS, preventing their migration to the anode.
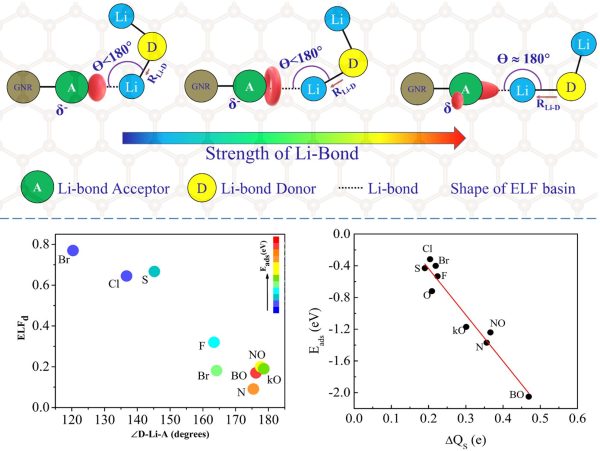
“Using first-principles calculations, we investigate a family of doped graphene nanoribbons (GNRs) for their suitability as cathode hosts in Lithium sulphur batteries. We probe the role played by the lone pairs of the dopants in confining the Lithium polysulfides (LiPS) in order to understand the mechanism of binding. Our results show that the Li-bond between the polysulfides and the doped GNRs is analogous to a hydrogen bond and also dipole-dipole interactions play a key role in anchoring the polysulfides. The charge lost by the sulphur atom of the polysulfide upon adsorption and shape of the lone pair basins and the value of ELF at the dopant position can provide a quick estimate of the strength of the bond. Significant contractions in the ELF profiles are also observed upon Li 2 S adsorption, further providing evidence for the H bond like nature of the Li-bond. Our results corroborate the fact that all acceptors suitable for hydrogen bond can be employed as suitable dopants for carbon-based cathode hosts in Li-S batteries.”
Dr Thapa and his PhD scholar conducted this research in collaboration with a Department of Physics and Research Centre, Lady Doak College, Madurai, 625002, Tamil Nadu, India. For their future research they are also working in the field of Li-ion battery. They are searching for new carbon allotrope structure which should have the ability to enhance the specific capacity and reduce the volume expansions as compared to the commercialized anode material.

