 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group pursuing PhD under him-Ms V.G.Vasavi Dutt and Mr Syed Akhil- have published a research article titled “Enhancement of Photoluminescence and Stability of CsPbX3 (X= Cl, Br, and I) Perovskite Nanocrystals with Phthalimide Passivation” in the Journal “Nanoscale” (The Royal Society of Chemistry, Impact Factor-7.8).
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group pursuing PhD under him-Ms V.G.Vasavi Dutt and Mr Syed Akhil- have published a research article titled “Enhancement of Photoluminescence and Stability of CsPbX3 (X= Cl, Br, and I) Perovskite Nanocrystals with Phthalimide Passivation” in the Journal “Nanoscale” (The Royal Society of Chemistry, Impact Factor-7.8).
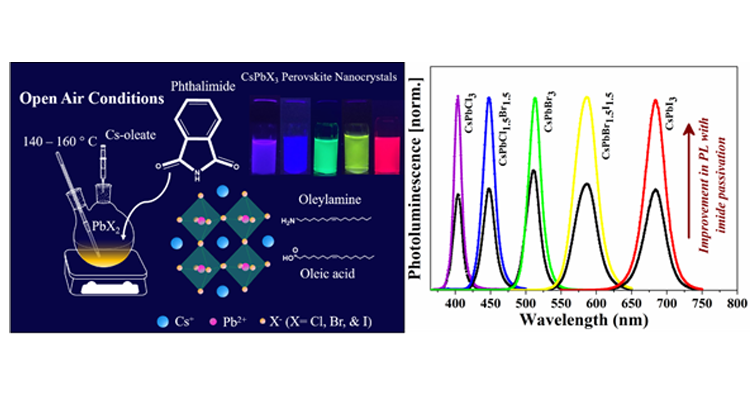
Caesium lead halide perovskite nanocrystals (CsPbX3 NCs) have been the flourishing area of research in the field of photovoltaic and optoelectronic applications because of their excellent optical and electronic properties. However, they suffer from low stability and deterioration of photoluminescence (PL) properties post-synthesis. One of the ways to minimize the surface defects in the surface treatment with suitable ligands is to achieve the NCs with superior PL properties for light-emitting applications.
In this article, Dr Mishra’s research group demonstrates that incorporating an additional ligand can further enhance the optical properties and stability of NCs. Here, we introduced phthalimide as a new surface passivation ligand into the oleic acid/oleylamine system in situ to get near-unity photoluminescence quantum yield (PLQY) of CsPbBr3 and CsPbI3 perovskite NCs. We observed, phthalimide passivation dramatically improves the stability of CsPbCl3, CsPbBr3, and CsPbI3 NCs under ambient light and UV light. The PL intensity is recorded for one year which showed a dramatic improvement for CsPbBr3 NCs. Nearly 11% of PL can be retained even after one year for phthalimide passivated samples, on the other hand, the PL of as-synthesized NCs completely diminishes in four months. CsPbCl3 NCs exhibit 3 times higher PL with phthalimide and retain 12% PL intensity even after two months while PL of as-synthesized NCs completely diminishes by then. Under continuous UV light illumination, the PL intensity of phthalimide passivated NCs is well preserved while the as-synthesized NCs exhibit negligible PL emission in 2 days. About 40% and 25% of initial PL is preserved for CsPbBr3 and CsPbCl3 NCs in the presence of phthalimide. CsPbI3 NCs with phthalimide exhibit PL even after 2 days while the PL is rapidly declined for as-synthesized NCs in the first 10 hours. The presence of phthalimide in CsPbI3 NCs could maintain stability even after a week while the as-synthesized NCs under transition to non-luminescent phase within 4 days.
Furthermore, blue, green, yellow, and red-emitting diodes by using CsPbCl1.5Br1.5, CsPbBr3, CsPbBr1.5I1.5, CsPbI3 NCs respectively are fabricated by drop-casting NCs onto blue LED lights which show the great potential of the use of these phthalimide passivated NCs in the field of display and light technologies.
Read the full paper here: https://pubs.rsc.org/en/content/articlelanding/2021/nr/d1nr03916d

