 The Department of Environmental Science is proud to announce that Dr Lakhveer Singh has published his paper titled, “Progressions in cathodic catalysts for oxygen reduction and hydrogen evolution in bioelectrochemical systems: Molybdenum as the next-generation catalyst” in a prestigious journal Catalysis Review with a high Impact Factor of 20.21.
The Department of Environmental Science is proud to announce that Dr Lakhveer Singh has published his paper titled, “Progressions in cathodic catalysts for oxygen reduction and hydrogen evolution in bioelectrochemical systems: Molybdenum as the next-generation catalyst” in a prestigious journal Catalysis Review with a high Impact Factor of 20.21.
The article is published in collaboration with NCL Pune, Hong Kong Baptist University, and VITO-Flemish Institute for Technological Research, Belgium.
Abstract of the Research
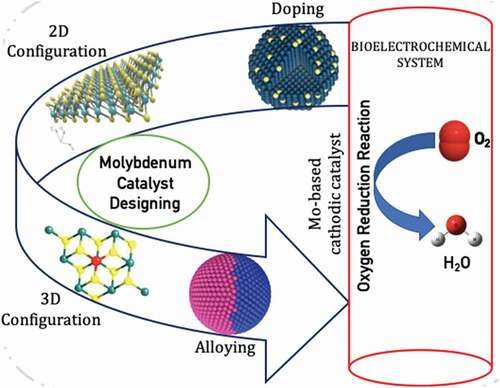
Oxygen reduction reactions (ORR) are unanimously a key factor of system performances in bioelectrochemical systems (BESs), low-temperature fuel cells, and generally in several electro-chemical platforms. Platinum (Pt)-based catalyst is the finest electrocatalyst for ORR in BESs; however, it is constrained by its low abundance, high price, and poor catalytic durability in an electrochemical setup for cathodic reaction kinetics. Molybdenum (Mo) with its multi-dimensional form as 2D and 3D layers and synergistic combination with other non-metals offers prospects of extraordinary performance as a low-cost metal-based ORR catalyst over the Pt in delivering enhanced ORR potential.
About the Research
This article throws light on the current requirements of sturdier catalyst material and thus provides a comprehensive review of the continuing efforts in exploring the possibility of Mo as a low-cost metal-based ORR catalyst for sustainable energy production.
Mo-based catalysts have been now widely used for their applications in environmental and energy-based catalysis due to the low cost of Mo, high stability, and excellent activity.
In the future, Dr Lakhveer Singh and his collaborators are working on overcoming limitations to fabricate durable, stable, and catalytically active micro/nanoscale two-dimensional MoS2-based cathodes at an industrial scale, commercial bioelectrochemical devices can be obtainable in future.

