Recent News
- Supercapacitor electrodes for enhanced energy storage April 18, 2022
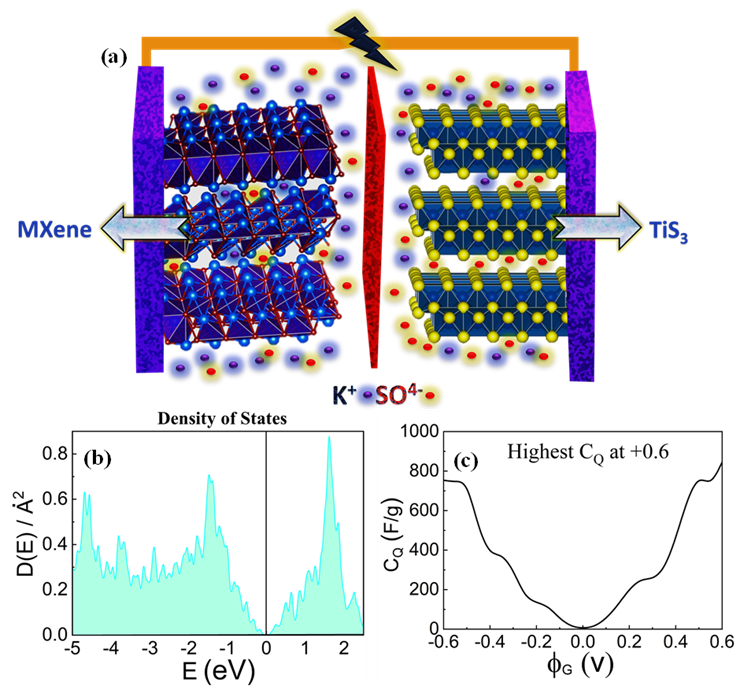
The Department of Physics is happy to announce that Prof Ranjit Thapa and his PhD Scholar Mr Samadhan Kapse have published a paper titled “Supercapacitor electrodes based on quasi-one-dimensional van der Waals TiS3 nanosheets: experimental findings and theoretical validation” in the Nature indexed journal ‘Applied Physics Letters’ having an impact factor of 3.79. The Paper is published in collaboration with Abhinandan Patra and Chandra Sekhar Rout from Jain University and Dattatray J Late from Amity University.
Abstract of the Research
To cease the ever-increasing energy demand, additional enthusiastic focus has been given to generate more sustainable energy from alternative renewable sources. The storage of these energies for future usage solely depends on the energy storage devices. A diversity of electrode materials based on two-dimensional (2D) transition metals and their derivatives have enticed the whole world owing to their tunable properties. Transition metal trichalcogenides (TMTCs- MX3 type) is the emergent class of 2D materials that gathered a lot of interest because of their quasi-one-dimensional anisotropic properties with the van der Waals force of attraction in between the layers. Herein, TiS3 being an MX3-type of material is preferred as the electrode for supercapacitor application with detailed experimental investigations and theoretical validation. The highest capacitance attained for TiS3 is found to be 235 F/g (105 C/g) at 5 mV/s with a battery type of charge storage mechanism. The asymmetric device is fabricated using Ti3C2Tx MXene nanosheets as negative electrode and a brilliant 91 % of capacitance retention is accomplished with an extensive potential window of 1.5 V. The investigational discoveries are substantiated by theoretical simulation in terms of the quantum capacitance assessment and charge storage mechanisms.
About the Research
In this work, a battery type TMTC material i.e., TiS3 has been synthesized and characterized by different analytical techniques such as Raman spectroscopy, FESEM and TEM to gain information on its structural and morphological aspects. The electrochemical performance was found to be promising by considering its good energy storage performance. High capacitance of 235 F/g (105 C/g) at 5 mV/s was achieved and the asymmetric supercapacitor devices disclosed outstanding cycling stability of 91 % over 6000 GCD cycles. In addition, the theoretical simulations also validated the experimental findings through the evaluation of the quantum capacitance. The higher conductivity, abundant electrochemical active sites, swift faradic redox kinetics and well-connected pathway for ion transfer characteristics pave the way for TiS3 to emerge as an eminent material for energy storage application in the long run.
Social Implications
Energy storage devices come into picture whenever there is a prerequisite of storing renewable energy. Among the numerous energy storage devices, batteries and ultracapacitors have acquired more popularity in nanotechnology and optoelectronics field. The high stability, accuracy, swift functionality, power density and reversibility are the key factors that have positioned ultracapacitors at the forefront of all energy storage devices. On the contrary, the low energy density and high cost of supercapacitor electrodes try to put them in the back seat of the wheels of the energy industry. Henceforth, in recent times the development of supercapattery (abbreviated for supercapacitor and battery) types of materials has become a way out which tie the aces like high specific power of supercapacitors with the high energy density of batteries. These materials exhibit capacitive or battery type behaviour on the basis of materials properties, electrolytic ions, design of the electrochemical cell. Due to these advantages and superior energy storage performance, the demand for this kind of material is growing.
Theoretical quantum capacitance is an important parameter to investigate the supercapacitor performance of low dimensional materials such as electrodes. This approach is highly cost-effective for the rapid screening of various materials for supercapacitor applications.
- Highly efficient catalysts of Ruthenium clusters on Fe3O4/MWCNTs for hydrogen evolution reaction March 22, 2022
“Highly Efficient Catalysts of Ruthenium Clusters on Fe3O4/MWCNTs for Hydrogen Evolution Reaction” is the latest paper published by Prof Ranjit Thapa, Professor of Physics at SRM university-AP and his PhD scholar, Mr Samadhan Kapse, in ‘New Journal of Chemistry’ having an Impact Factor of 3.591.
In this work, the chemical co-precipitation technique is adopted to produce Fe3O4 nanoparticles under an inert atmosphere and was utilized for HER studies. The Ru nanoparticles were profitably deposited over Fe3O4/MWCNTs GC electrode using electrochemical deposition technique. The superior HER activity was achieved on Ru/Fe3O4/MWCNTs in 0.1 M H2SO4 aqueous media. We have demonstrated that the synthesized electrocatalyst offers a low overpotential of 101 mV to achieve a current density of 10 mA cm-2 towards the hydrogen evolution reaction. It displays long-term durability, exceptional cyclic stability even after 1000 cycles. DFT calculations imply that the availability of both octahedral and tetrahedral sites in Ru/Fe3O4/MWCNTs with low overpotential is more efficient towards HER. It is emphasized that a low percentage of ruthenium in the prepared catalyst can be substituted as a promising HER catalyst for sustainable energy technologies.
Abstract of the paper
Producing molecular hydrogen (H2) using water provides a sustainable approach for developing clean energy technologies. Herein, we report highly active ruthenium clusters (Ru) supported on iron oxide (Ru/Fe3O4) and Fe3O4/multi-walled carbon nanotubes (Ru/Fe3O4/MWCNTs) by simple electrochemical deposition in a neutral aqueous medium. The supported catalyst exhibits good hydrogen evolution reaction activity (HER) in an acidic environment. Cyclic voltammograms (CV) in potassium ferrocyanide (K4[Fe(CN)6]) confirm MWCNTs enhance the electron transfer process by decreasing the redox formal potential. The overpotential of Ru/Fe3O4/MWCNTs and Ru/Fe3O4 electrocatalysts versus reversible hydrogen electrode (RHE) was found to be 101 mV and 306 mV to reach a current density of 10 mA cm-2 . As prepared, the catalyst displays good stability and retain its HER activity even after 1000 cycles. Further, the stability of Ru/Fe3O4/MWCNTs was studied using chronopotentiometric (CP) technique for 12 hrs and found negligible loss in the catalytic activity towards HER. To explore the role of Ru and underneath MWCNTs to improve the catalytic performance of Fe3O4, density functional theory (DFT) calculations were carried out. DFT calculations indicate the octahedral site of Ru/Fe3O4 favours HER with low overpotential. However, Ru/Fe3O4/MWCNTs is more efficient towards HER could be due to the availability of both octahedral and tetrahedral catalytic sites.
Social implications of the research
Renewable energy generation is of greater importance in the present circumstances. This is caused by the depletion of non-renewable energy sources like fossil fuels and other hydrocarbon deposits and the release of greenhouse gases into the atmosphere. Hydrogen has gained considerable interest as an energy storage and energy carrier because of its high energy density (146kJ/g). Furthermore, its lightweight, profusion nature and the release of water during its combustion eliminate environmental pollution and thus contribute to defeating the global energy crisis. Also, hydrogen is an important and ideal energy source to develop fuel cells. A number of methods have been explored to generate molecular hydrogen. Among them, water electrolysis is a promising technology for generating high-purity hydrogen from water. An excellent electrocatalyst is obligatory to liberate hydrogen gas effectively from water. A higher HER activity is known to be obtained from platinum (Pt) and Pt-based catalysts. Given its high cost and low surplus, it limits expansion to the industrial scale. Over the few past decades, lots of efforts have been made by many research teams to find out alternative catalysts to substitute Pt electrodes.
The paper is published in collaboration with Shwetha Kolathur Ramachandra, Doddahalli Hanumantharayudu Nagaraju, and Shivanna Marappa; School of Applied Sciences, REVA University, Bangalore-560064, Karnataka, India. According to the research group, the density functional theory can boost the searching process of highly promising electrocatalysts for hydrogen evolution reactions in renewable energy generation.
- Chanakya Karra admitted to PhD at Purdue University, USA March 21, 2022
 Once you are a part of SRM University-AP, we ensure that your future is secured! With the guidance of Dr Sujith Kalluri, Assistant Professor, Electronics and Communication Engineering, Mr Chanakya wends his way to Purdue University, USA, a world-renowned research university, for doing his PhD. He secured admission with a full tuition fee waiver and teaching assistantship. Chanakya Karra spent his two years DST-SERB JRF position at SRM AP and has made remarkable contributions to SRM-Amararaja Centre for Energy Storage Devices.
Once you are a part of SRM University-AP, we ensure that your future is secured! With the guidance of Dr Sujith Kalluri, Assistant Professor, Electronics and Communication Engineering, Mr Chanakya wends his way to Purdue University, USA, a world-renowned research university, for doing his PhD. He secured admission with a full tuition fee waiver and teaching assistantship. Chanakya Karra spent his two years DST-SERB JRF position at SRM AP and has made remarkable contributions to SRM-Amararaja Centre for Energy Storage Devices.DST-SERB JRF position helped Chanakya resume his research career, which had a pause for over a year. “It fills me with immense joy to see the SRM-Amararaja Centre for Energy Storage Devices shape up with every possible equipment to conduct research on batteries. Kudos to the management and the efforts of the faculty associated with the centre,” says Mr Chanakya. He further mentioned that the research work conducted at SRM-Amara Raja Centre enabled him to write over three papers that catapulted his chances of admission.
“I would urge the students to make the best use of the opportunities available at SRM-AP and discuss their plans with the faculty. I am sure new avenues will open with the mentoring of world-class faculty at SRM”, says Mr Chanakya to the junior batches of students aspiring for a research career.
Mr Chanakya expressed his gratitude to the faculty members associated with Amararaja Centre for Energy Storage Devices- Dr Pardha Saradhi Maram, Associate Professor, Chemistry, Dr Surfarazhussain S Halkarni, Assistant Professor, Mechanical Engineering, Dr Laxmi Narayana Patro, Assistant Professor, Physics, and others.
Continue reading → - A novel method of synthesising 2D transition metal oxide layers March 15, 2022
Patent application No: 202241005220
Publication date: 11/02/2022
Title: Two-Dimensional Transition Metal Oxide Layers and a method for their Synthesis
Inventors: Dr. Jatis Kumar Dash, Shaik Md. Abzal, Kurapati Kalyan, Sai Lakshmi Janga
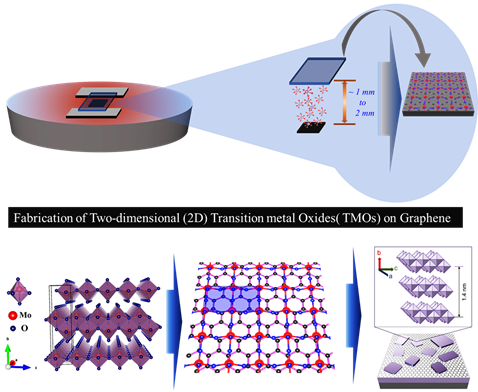
Department of Physics, SRM-University-AP, Andhra PradeshThe Department of Physics is glad to announce that Dr Jatis Kumar Dash and his PhD Scholars Shaik Md. Abzal, Kurapati Kalyan and Sai Lakshmi Janga have got their patent “TWO-DIMENSIONAL TRANSITION METAL OXIDE LAYERS AND A METHOD FOR THEIR SYNTHESIS” published on February 11, 2022.
About the Patent
Extensive use of portable electronic products and the rapidly growing commercial markets in smart electric appliances have created a seemingly high demand for flexible, wearable high-performance photoelectric devices and energy storage technology. In the search for new materials to meet these criteria, one promising solution may be the two-dimensional (2D) material heterostructures, assembled by stacking different conventional 2D materials (for example, graphene, transition metal oxides, carbides, and chalcogenides) in hetero-layered architectures.
These 2D materials stackings are ultrathin layered crystals that show unusual physicochemical properties at few-atom thickness. These 2D heterostructures offer several key advantages for the next-generation devices such as (i) atomically thin 2D nanosheets provide a larger surface area due to complete exposure of the surface atoms, (ii) the edge sites in 2D nanosheets are chemically more reactive than their basal planes and the open gaps enable the intercalation of electrolyte ions and (iii) the high mechanical strength and flexibility at atomic dimensions allow them to be used in the next-generation wearable electronics.
But the growth and stacking of 2D materials is always a challenge. Also, the existing growth tools are complex and expensive. Here, at SRM University-AP, we have fabricated the large-area ultra-thin 2D transition metal oxide (TMO) layers using an easy and cost-effective method. In addition, these 2D TMO layers are further integrated to different other 2D materials for their use in nano-electronic devices. Our work shows the great potential of ultra-thin TMOs in 2D-material-based flexible electronics.
Social Implication
2D materials are the prime candidates for making flexible, wearable, foldable and transparent self-powered smart electronic devices. The next-generation smart electronic devices will be made of 2D materials heterostructures which will need less operating power, less consumption of materials and will have ultimate scalability.
The team is also in the process of optimization and aims to make prototype flexible 2D supercapacitors, photodetectors, ultrathin transistors, and various sensors.
- Eco-friendly and economic production of Ammonia February 1, 2022
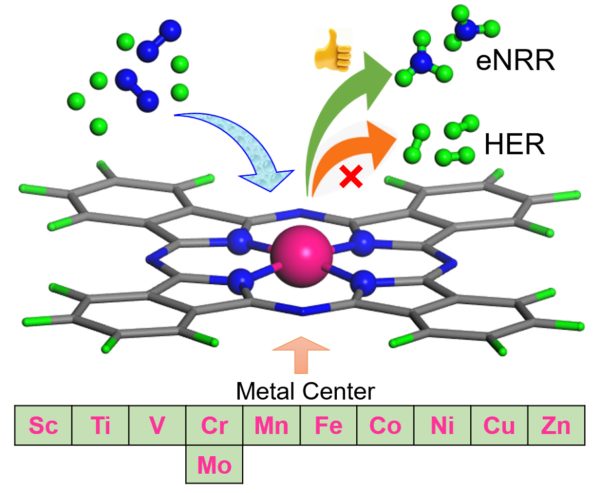 SRM Univeristy-AP is proud to announce that Prof. Ranjit Thapa, Department of Physics, has obtained a prestigious SERB-DST grant of Rs. 32 lakhs for a period of three years for his project, “Design Principle of Single Atom Catalyst for Nitrogen Fixation over HER: Energy Parameter, Electronic Descriptor and Database”.
SRM Univeristy-AP is proud to announce that Prof. Ranjit Thapa, Department of Physics, has obtained a prestigious SERB-DST grant of Rs. 32 lakhs for a period of three years for his project, “Design Principle of Single Atom Catalyst for Nitrogen Fixation over HER: Energy Parameter, Electronic Descriptor and Database”.Ammonia (NH3) is the prime source of fertilizers and an important carrier of energy too. Ammonia can be stored in its chemical form for a long and it is easy to transport. So now researchers are looking forward to using ammonia in place of hydrogen as an energy source. But the production of ammonia with existing techniques needs more energy compared to the energy it stored in its chemical bond. So, an alternative process that is environmentally friendly and cost-effective is needed to be in place.
In 2019 the global production capacity of ammonia is 235 million metric tons and will increase to 290 million metric tons by 2030. The importance of ammonia is due to its application in broad and diverse fields, such as fertilizers, textiles, pharmaceuticals, and is a carbon-free energy carrier. The Haber-Bosch process is used for the synthesis of ammonia (NH3) from N2 and H2 using Fe based catalyst. But the process emits carbon dioxide (CO2) (1.5 tons of CO2/tons of NH3 production) requires high pressure and temperature and consumes around 2% of the global supply of energy. Electrocatalytic N2 fixation (N2 + 6H+ + 6e− → 2NH3) showed great potential due to the possible use of atmospheric nitrogen and hydrogen derived from water through electrolysis and in mild conditions. However, the slow kinetics of N2 adsorption, splitting of the strong N≡N bond are the challenges for the electrocatalytic NRR process. In the electrocatalytic NRR process, the fast reaction kinetics of hydrogen evolution reaction is the greatest obstacle. To solve these challenges, the search for various types of catalysts is on the roll.
To date, trial and error methods have been used to synthesize the catalysts for the electrocatalytic NRR process. Thanks to the rapid development of density functional theory-based computational methods, the intermediate steps during NRR can be identified at the atomic level, the underlying principles can be understood, and a large space of catalysts can be checked for efficient NRR within a limited time. Without understanding the correct electronic structure of SAC and its correlation with the overpotential of NRR and defining the correct energy parameter to define “NRR over HER” and “N2 binding over H binding free energy”, we can never design the best catalyst cost-effectively. We will address these problems through this project’s objectives.
The project will help to design the best single-atom catalyst for the reduction of nitrogen (from the air) through the electrocatalytic process and convert it into ammonia. The designed catalyst can be synthesis by the industry and can be used for NRR.
This project will help a step forward towards more ammonia production for the uses in the agriculture sector, energy sector, and related sector.