Recent News
- Enhanced charge transport behaviour of protein-metal nanocluster hybrid June 14, 2022
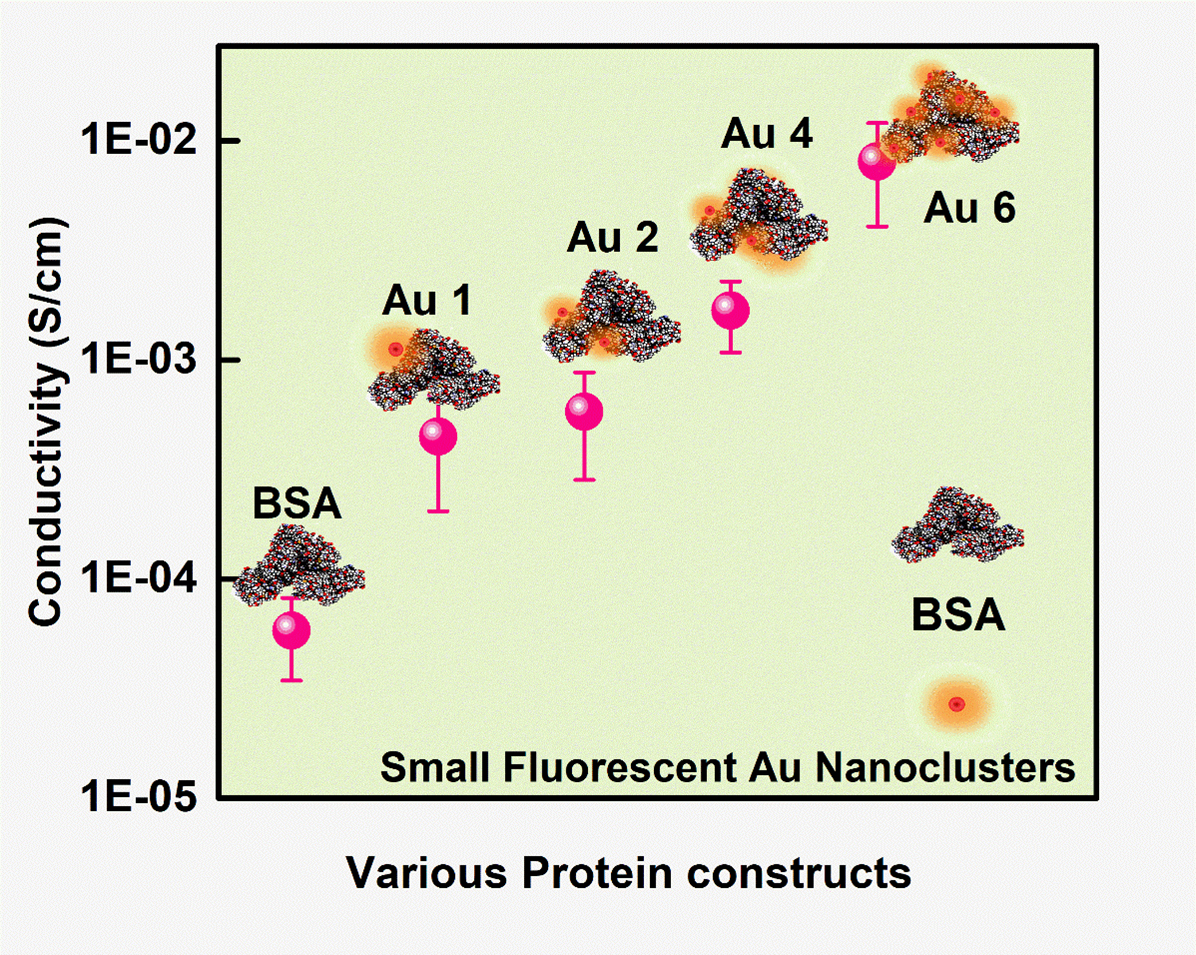
Proteins are the most vital life forms which maintain close coordination with almost living activities through their biological functions. Nevertheless, in most cases, proteins suffer from low charge (electron) transfer efficiency as they are mainly made of insulating organic molecules. The interdisciplinary research publication, of Dr Sabyasachi Mukhopadhyay and Dr Sabyasachi Chakrabortty from the Department of Physics & Department of Chemistry respectively, along with their PhD scholars: Ms Ashwini Nawade, Mr Kumar Babu Busi and Ms Kunchanapalli Ramya, envisions the molecular-level understanding of the charge transport behaviour of various protein-metal nanocluster hybrid.
The article titled ‘“Improved Charge Transport across Bovine Serum Albumin – Au Nanoclusters’ Hybrid Molecular Junction” was featured in the prestigious Q1 journal ACS Omega (IF: 3.512), published by the ‘American Chemical Society’. They successfully incorporated Gold Nanoclusters inside the protein backbone leading to an increase in their conductivity. This will provide new avenues for the rational design of bioelectronic devices with optimized features. The BSA-Au cluster has been a promising model for bioelectronic functionalities. With an increase in their current carrying capacity, they can be used for many more applications, especially as the interface between tissue and organ in biocompatible devices. The research team is also planning to work with various protein dopants to understand their charge transport mechanism. These studies will help in using the protein for various applications mainly in bioimplants or biosensors for drug testing and diagnostics purposes.
Abstract of the Research
Proteins, a highly complex substance, have been the essential element in the living organism where various applications are envisioned due to their biocompatible nature. Apart from protein’s biological functions, contemporary research mainly focuses on their evolving potential associated with nanoscale electronics. Here, we report one type of chemical doping process in model protein molecules (BSA) to modulate its electrical conductivity by incorporating metal (Gold) nanoclusters on the surface or within it. The as-synthesized Au NCs incorporated inside the BSA (Au 1 to Au 6) were optically well characterized with UV-Vis, time-resolved photoluminescence (TRPL), X-ray photon spectroscopy, and high-resolution transmission electron microscopy techniques. The PL quantum yield for Au 1 is 6.8% whereas Au 6 is 0.03%. In addition, the electrical measurements showed ~10-fold enhancement of conductivity in Au 6 where maximum loading of Au NCs was predicted inside the protein matrix. We observed a dynamic behaviour in the electrical conduction of such protein-nanocluster films, which could have real-time applications in preparing biocompatible electronic devices.
Continue reading → - Neodymium-doped bismuth ferrite thin films for random access memory applications June 13, 2022
 A paper titled “Study on ferroelectric polarization induced resistive switching characteristics of neodymium-doped bismuth ferrite thin films for random access memory applications” has been published by Dr Pranab Mandal, Assistant Professor of Physics and his PhD student, Ms K N Malleswari in the journal ‘Current Applied Physics’ having an Impact Factor of 2.480.
A paper titled “Study on ferroelectric polarization induced resistive switching characteristics of neodymium-doped bismuth ferrite thin films for random access memory applications” has been published by Dr Pranab Mandal, Assistant Professor of Physics and his PhD student, Ms K N Malleswari in the journal ‘Current Applied Physics’ having an Impact Factor of 2.480.Doi: https://doi.org/10.1016/j.cap.2022.04.013
Abstract
Resistive random-access memory (ReRAM) devices are based on the resistance switching (RS) effect. Such RS devices have recently attracted significant attention due to their potential application in realizing the next-generation non-volatile memory (NVM) devices. The present work reports on resistive switching (RS) characteristics of Neodymium (Nd)-doped bismuth ferrite (BFO) layers. The Nd (2–10 at%) doped BFO thin film layers were deposited using a spray pyrolysis method. The structural analysis reveals that a higher Nd doping concentration in BFO leads to significant distortion of the prepared Nd: BFO thin films from rhombohedral to tetragonal characteristics. The morphological analysis shows that all the deposited Nd: BFO thin films have regularly arranged grains. The X-ray photoelectron spectroscopy (XPS) analysis reveals that the prepared Nd: BFO thin films have a higher Fe3+/Fe2+ ratio and fewer oxygen vacancy (VO) defects which enrich the ferroelectric characteristics in Nd: BFO layers. The polarization-electric field (P-E) and RS characteristics of the fabricated Nd: BFO-based RS device were examined. It was observed that the Nd (7 at%) doped BFO RS device shows large remnant polarization (P r) of 0.21 μC/cm2 and stable RS characteristics.
Research in brief
Non-volatile resistive random access memory (RRAM) are future generation random access memory device with potential benefits such as high operational speed (nanoseconds read and write time), non-volatility, high endurance scalability and low power consumption [Namnoscale Research Lett., 15, 90, 2020]. Here in this work, we presented the resistive switching characteristics of a multiferroic material namely Nd-doped BiFeO3 material. The device shows stable resistive switching characteristics.
Practical implementation/social implications
Researchers in this field are focusing to overcome challenges of high operation current, lower resistance ratios, and reliability issues [Namnoscale Research Lett., 15, 90, 2020]. While several prototype RRAMs have been developed by other groups, future memory applications would require overcoming the challenges mentioned above.
Collaboration
The work has been conceptualized by Dr Amiruddin at Crescent Institute of Science and Technology, Chennai; and Dr Pranab Mandal and Ms Malleswari provided inputs on ferroelectric polarization – electric field (P – E) measurement and drafting.
Continue reading → - The potential applications of NdNiO3 June 7, 2022
Research at the Department of Physics is currently exploring the potential applications of NdNiO3. Recently, Professor Ranjit Thapa, and his Ph D student, Mr Deepak S Gavali published the paper, Low-Temperature Spin-Canted Magnetism and Bipolaron Freezing Electrical Transition in Potential Electron Field Emitter NdNiO3 in the journal ACS Applied Electronic Materials, with an Impact Factor of 3.314. This work is done in collaboration with the Department of Physics and Astronomy, National Institute of Technology Rourkela, Rourkela, Odisha, India.
About the research
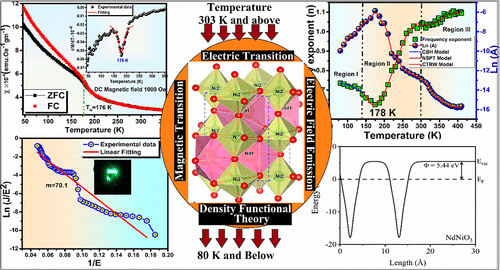 In this work, NdNiO3 nanoparticles are synthesized by sol-gel auto-combustion techniques, and its primary characterization related to structural and surface morphological analysis is carried out by X-Ray Diffraction (XRD), Fourier Transforms Infrared Spectroscopy (FTIR), Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray spectroscopy (EDX), and Transmission Electron Microscopy (TEM) techniques. The research is focused on magnetic phase transition below Curie temperature (TN) ∼176 K, and the magnetic susceptibility indicates a weak antiferromagnetic ordering at low temperature. Different ac conduction mechanisms, that is, Correlated Barrier Hopping (CBH), Continuous-Time Random Walk (CTRW) conduction model, and Non-overlapping Small Polaron Tunneling (NSPT), are introduced to interpret its electrical transport behavior near, above, and below TMI ∼178 K. Using first principles and Density of States (DOS) calculation, the researchers have characterized the electronic and magnetic ground state of NdNiO3 at room temperature. It exposed the overlapping of conduction and valence band at room temperature, and the degree of hybridization between Ni 3d and O 2p is very high compared to Nd 5d states. The work function is also calculated for a few-layer NdNiO3 to estimate the field enhancement factor (β), which plays a crucial role in the practical application of a field emitter.
In this work, NdNiO3 nanoparticles are synthesized by sol-gel auto-combustion techniques, and its primary characterization related to structural and surface morphological analysis is carried out by X-Ray Diffraction (XRD), Fourier Transforms Infrared Spectroscopy (FTIR), Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray spectroscopy (EDX), and Transmission Electron Microscopy (TEM) techniques. The research is focused on magnetic phase transition below Curie temperature (TN) ∼176 K, and the magnetic susceptibility indicates a weak antiferromagnetic ordering at low temperature. Different ac conduction mechanisms, that is, Correlated Barrier Hopping (CBH), Continuous-Time Random Walk (CTRW) conduction model, and Non-overlapping Small Polaron Tunneling (NSPT), are introduced to interpret its electrical transport behavior near, above, and below TMI ∼178 K. Using first principles and Density of States (DOS) calculation, the researchers have characterized the electronic and magnetic ground state of NdNiO3 at room temperature. It exposed the overlapping of conduction and valence band at room temperature, and the degree of hybridization between Ni 3d and O 2p is very high compared to Nd 5d states. The work function is also calculated for a few-layer NdNiO3 to estimate the field enhancement factor (β), which plays a crucial role in the practical application of a field emitter.Practical implications
The additional novelty of the present work is to explore the potential application of NdNiO3 as an efficient field emitter and controlled electron/X-ray sources in a flat panel display, microwave vacuum electronic devices, electron microscopy/ lithography, and so forth. To eject conducting electrons from the metal/semiconducting surface by a quantum mechanical tunneling process, sufficient energy is required in terms of the applied electric field (∼106 to 107 V/cm) to overcome the potential barrier at the vacuum−metal interface. The potential difference between the Fermi level (Ef ) of the metal surface to vacuum is known as the work function (Φ). It depends on material characteristics and plays an essential role in field enhancement capability. The primary requirement for efficient field emitters is high aspect ratios (i.e., field enhancement factor), inferior turn-in field, low work, function, etc. Researchers have examined various classes of materials for efficient field emitter electrodes, such as (i) carbonaceous materials like graphene and carbon nanotube, (ii) various 1D and 2D metal oxide and transition metal dichalcogenides like ZnO, MnO2, In2O3, WS2, WSe2, MoS2, PdSe2, etc., (iii) inorganic semiconductors like SiC and Si, and (iv) wide band gap semiconducting compounds GaN, AIN, and so on. The field emission properties of rare earth nickelates (RNiO3; R = La, Gd, Nd, Sm, etc.) with an exciting room temperature metallic nature have not been examined.
Continue reading → - Applying the Pareto principle in disordered systems June 6, 2022
The Department of Physics is glad to announce that Dr Soumyajyoti Biswas, Assistant Professor, has published a paper titled ” Near universal values of social inequality indices in self-organized critical models” in the journal Physica A: Statistical Mechanics and its Applications having an impact factor of 3.263. This research was done in collaboration with Prof S S Manna of S N Bose National Center for Basic Sciences and Prof B K Chakrabarti of Saha Institute of Nuclear Physics.
It is well known that wealth invariably accumulates only in a few hands while a majority of the world continues to remain poor. In economics, it is quantified in Pareto’s 80-20 law (20% of people possess 80% of wealth) or ‘The Law of the Vital Few’. This research reveals that the implication of this law goes far beyond the socio-economic systems. It is also a crucial indicator of the onset of critical phenomena in a wide class of physical systems.
It has been observed that in the dynamics of disordered systems, such as fracture and breakdown of solids, slowly increasing the external force produces acoustic emissions (crackling noise), the sizes of which follow Pareto-like behaviour (most noises are weak, only a few are strong that results in the breakdown). Quantifications of these “inequalities” in these physical systems reveal some universal characteristics in a wide class of models, known as self-organized critical systems.
The main implication of this observation lies in predicting catastrophic breakdown in disordered systems. Applications of these inequality measures, which are traditionally in the domain of social sciences, have proved to be immensely useful in identifying the approaching breakdown points in the models of disordered systems. Given that the methods are applicable to a wide variety of models, the 80-20 law has the potential for a wide range of applications. Dr Biswas and his PhD student Diksha are currently working with a team in Spain on experimental data and studying these inequalities in real systems.
Continue reading → - Reconceiving the building blocks of the Universe June 2, 2022
The research at the Department of Physics is currently focusing on developing new theoretical frameworks to revamp the fundamental concepts that describe the origin of the universe. Assistant Professor Dr Amit Chakraborty has published a paper titled Revisiting Jet Clustering Algorithms for New Higgs Boson Searches in the Hadronic Final States in the European Physical Journal C, with an Impact Factor of 4.59.
Abstract
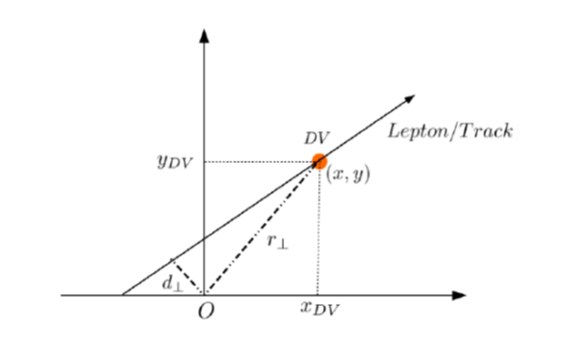 Displaced signatures originating from the pair production of a supersymmetric particle, called sneutrino, at the Large Hadron Collider (LHC) are studied. The theoretical model considered in this work is the Next-to-Minimal Supersymmetric Standard Model supplemented with right-handed neutrinos triggering a Type-I seesaw mechanism. The research has shown how such signatures can be established through a heavy Higgs portal when the sneutrinos are decaying to charged leptons and charginos giving rise to further leptons or hadrons. The research also illustrated how the Yukawa parameters of neutrinos can be extracted by measuring the lifetime of the sneutrino from the displaced vertices, thereby characterising the dynamics of the underlying mechanism of neutrino mass generation.
Displaced signatures originating from the pair production of a supersymmetric particle, called sneutrino, at the Large Hadron Collider (LHC) are studied. The theoretical model considered in this work is the Next-to-Minimal Supersymmetric Standard Model supplemented with right-handed neutrinos triggering a Type-I seesaw mechanism. The research has shown how such signatures can be established through a heavy Higgs portal when the sneutrinos are decaying to charged leptons and charginos giving rise to further leptons or hadrons. The research also illustrated how the Yukawa parameters of neutrinos can be extracted by measuring the lifetime of the sneutrino from the displaced vertices, thereby characterising the dynamics of the underlying mechanism of neutrino mass generation.Explanation of the research
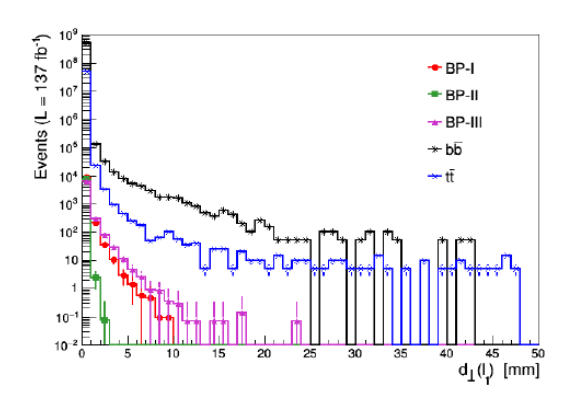 The Standard Model of Particle Physics is currently the remarkably successful theory to describe the basic building blocks of the universe and their interactions with the three fundamental forces of nature. Despite its success at explaining the universe, the Standard Model does have several limitations. For example, how neutrinos get their mass, why the mass spectrum of the different elements of SM fermions, namely quarks and leptons, are so hierarchical, why the Higgs boson mass is so low, etc. The primary research is to understand these issues and then propose theoretical models which circumvent these shortcomings of SM and provide signatures that can be tested in the ongoing or future proposed experiments.
The Standard Model of Particle Physics is currently the remarkably successful theory to describe the basic building blocks of the universe and their interactions with the three fundamental forces of nature. Despite its success at explaining the universe, the Standard Model does have several limitations. For example, how neutrinos get their mass, why the mass spectrum of the different elements of SM fermions, namely quarks and leptons, are so hierarchical, why the Higgs boson mass is so low, etc. The primary research is to understand these issues and then propose theoretical models which circumvent these shortcomings of SM and provide signatures that can be tested in the ongoing or future proposed experiments.For this research project, Dr Amit Chakraborty have collaborated with Particle Physics Department, STFC Rutherford Appleton Laboratory, UK and School of Physics and Astronomy, University of Southampton, UK. His broad research interest is to perform theoretical studies of physics beyond the Standard Model (BSM) in particular, collider search strategies and prospects of different BSM models at the Large Hadron Collider (LHC) and future proposed collider experiments. He aims to build new theoretical models, develop new techniques/tools, and devise new search strategies to improve our knowledge of the standard model as well as BSM physics processes.
Dr Amit Chakraborty’s future research topics include Higgs Boson Physics and Beyond Standard Model Physics Phenomenology, Dark Matter at the Colliders, Interpretable Machine Learning techniques in BSM Physics, and Ultra-light particles and Physics Beyond the Colliders.
Continue reading →

