Recent News
- SERB- SURE Grants: 10 projects worth 2.50 crores awarded to SRM AP May 16, 2023

The faculty of SRM University-AP have been awarded 10 projects worth 2.50 crores from the Science and Engineering Research Board (SERB-SURE). Department of Science and Technology (DST) received a total of 2000 proposals, of which 466 were sanctioned. Among the 466 projects, 151 projects were awarded to Private Universities. Of the 151 projects approved to state private universities and colleges throughout India, the five-and-a-half-year young varsity was awarded 10 projects. 10 professors from various Science and Engineering Departments brought this incredible achievement to the university.
SERB-SURE is a research grant scheme initiated by the Science and Engineering Research Board (SERB) in India to provide financial support to young researchers in the early stages of their careers. The grants are intended to support research in basic and applied sciences, engineering, and technology and is typically granted for a period of three years.The SERB-SURE scheme is one of several initiatives by SERB to promote scientific research in India and support the development of a strong research community in the country.
“It is a milestone achievement that resonates with the University’s unparalleled commitment for excellence. We are striving towards research-intensive learning to build cutting-edge innovation for a transformative tomorrow”, commented Vice Chancellor, Prof. Manoj K Arora. The Executive Director-Research of SRM Group, Prof. Narayana Rao said that, “SRM University-AP has travailed hard to achieve the world-class scientific temperament that we now advocate, and this achievement is a testimonial recognition of all our efforts.” The prestigious grants were sanctioned to the faculty in the on-going domains of Quantum Kinetic Approach, Antimicrobial Resistance (AMR) Profiling and Changing of Hydroclimatic conditions in Bay of Bengal among 7 others.
Dean-SEAS, Prof. Ranjith Thapa said, “These research could be path-breaking and could offer a solution to many of the societal difficulties.” Prof. Jayaseelan Murugaiyan, Dr Sandeep Singh and Dr Pitchaiah Cherukuri of the Department of Biological Sciences; Dr Sabyasachi Chakrabortty, Dr V S Baswanth Oruganti of the Department of Chemistry; Dr Debabrata Pramanik, Dr Ravi Kumar and Dr Pankaj Bhalla of the Department of Physics ; Dr Sandeep Kumar Verma of the Department of Mathematics; Dr Uma Maheswar Arepalli of the Department of Civil Engineering; and Dr Kousik Das of the Department of Environmental Science and Engineering were awarded the grants.
Continue reading → - Doctoral scholar secures visiting fellowship November 9, 2022

Exposure to international research opportunities promotes empirical learning at an impeccable level. International research ventures aid scholars to explore novel research avenues enabling a transformative progress for society through the field of science. The Department of Chemistry is glad to announce that Ms Jayasree K, PhD scholar, has been accepted for Short-Term Research Internship (STRI) for a period of six months from the Research Center of Environmental Medicine, Kaohsiung Medical University, Taiwan.
Ms Jayasree has been elevated in receiving the offer and delightfully keen on the new avenues she could explore through this opportunity. She is currently working in the field of surface-enhanced Raman spectroscopy (SERS). In this particular research area, her major research objective is the design and development of a novel SERS substrate for food and bioanalysis.
“My internship mentor, Prof. Vinoth Kumar, KMU University is an expert in mass spectroscopy and High-performance liquid chromatography (HPLC). Therefore, I have an option to hyphenate the Raman technique along with mass spectroscopy which leads Raman research to the next level for various applications”, commented Ms Jayasree on this incredible opportunity.
Her internship at Kaohsiung Medical University (KMU) is based on the motive of research on food and environmental toxicity which would provide guidance on her first research project in the field of food analysis.
She has offered her sincere gratitude to her supervisor, Dr Rajapandiyan JP, Department of Chemistry for his constant support and advice from the application process to proposal writing, experimental planning etc. She also thanked SRM University- AP in providing support through the process and extending travel allowance and guidance.
Ms Jayasree utilizes this great opportunity to explore and discover herself, developing both personally and professionally. Through this internship she hopes to learn new skills, expand her knowledge in the field of research and explore career options in Taiwan.
Continue reading → - Highly-stable amine-free CsPbBr3 PNCs for display applications September 22, 2022
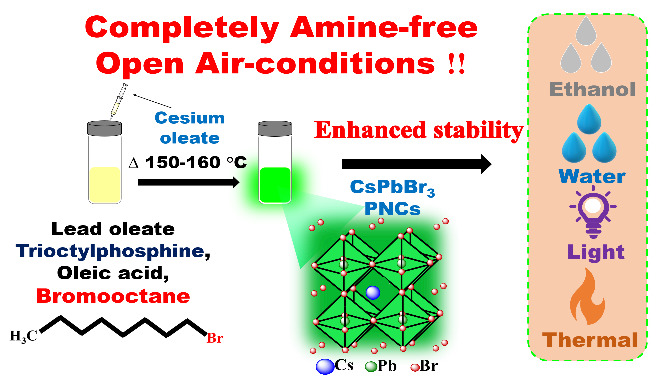
The Department of Chemistry is glad to announce that Dr Nimai Mishra, Assistant Professor, along with his research group comprising PhD scholars, Syed Akhil, Manoj Palabathuni, Subarna Biswas, Rahul Singh, have published an article titled Highly-Stable Amine-Free CsPbBr3 Perovskite Nanocrystals for Perovskite-Based Display Applications in the journal ACS Applied Nano Materials published by the American Chemical Society, having an impact factor of 6.14.
Colloidally synthesised cesium lead halide (CsPbX3; X=Cl, Br, and I) perovskite nanocrystals (PNCs) often suffer from poor ambient and environmental stability conditions, limiting their practical applications. The commonly used surfactant oleylamine is converted to oleylammonium cation, which pulls out the halide anion from the PNCs surface, thus disrupting the nanocrystal’s structural integrity and stability.
The research group has developed a simple, completely amine-free colloidal synthesis with a hot injection method in open-atmospheric conditions and introduced bromooctane as a bromine precursor to overcome the above issues. These, as synthesized amine-free PNCs, showed a photoluminescence quantum yield (PLQY) of around 60 %, and the size of PNCs is ~25 nm. Moreover, these amine-free PNCs were highly stable in the colloidal solution and thin films for more than five months in ambient conditions, with 66% of its initial PLQY.
In addition, these PNCs have shown exceptional stability under different environmental conditions, with 44 % of initial PL even after 6 hours of water treatment and 28 % of initial PL under ethanol treatment for 120 minutes. Furthermore, it has exhibited excellent photostability for 96 hours and retained 36 % of its initial PL under ceaseless UV light irradiation at 365 nm (8 W/cm2). Additionally, these PNCs have good stability upon heat treatment and maintained 34 % of initial PL upon heating up to 90 ºC.
The research team has also successfully fabricated the green-emitting down-conversion LED using these amine-free PNCs. Thus, they visualize that these amine-free CsPbBr3 PNCs are perhaps the ideal candidates for perovskite-based display applications.
Continue reading →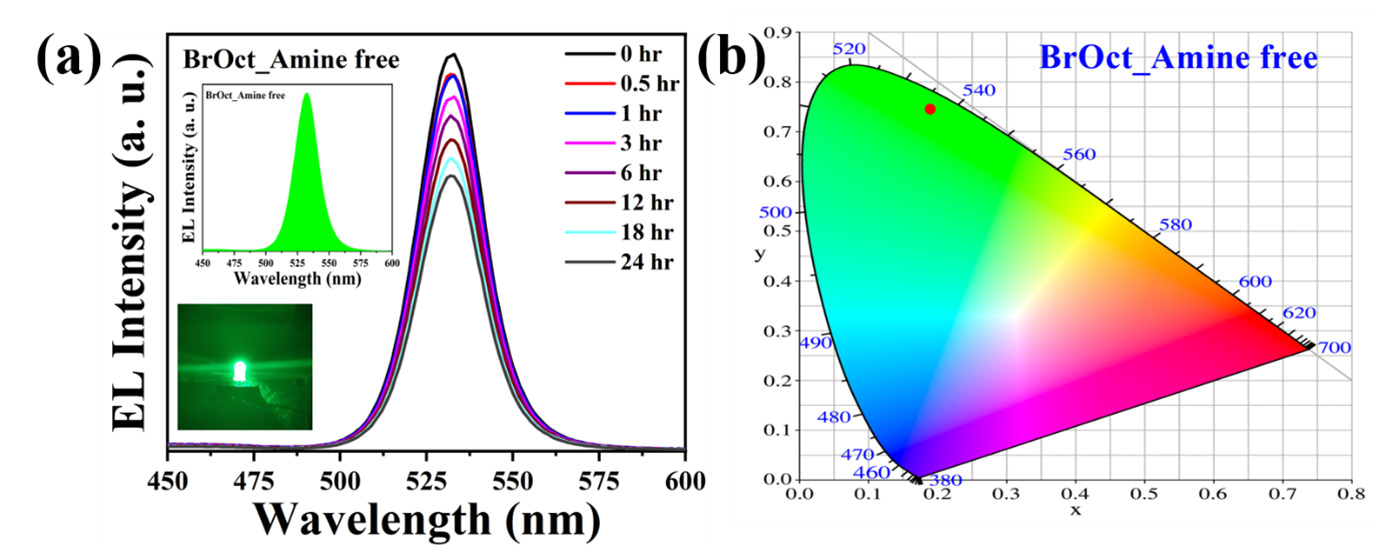
- Molecular design to store solar energy September 22, 2022
 India has an ambitious target of achieving 300 GW of solar power by 2030. Conventional methods for producing solar power involve absorbing sunlight by a molecule and converting it directly into electricity. This is possible only during the daytime when sunlight is available. An interesting and complementary prospect is storing the absorbed solar energy by converting it into a different form of energy, such as chemical energy, which can then be transformed into electrical energy when sunlight is not available during the night-time.
India has an ambitious target of achieving 300 GW of solar power by 2030. Conventional methods for producing solar power involve absorbing sunlight by a molecule and converting it directly into electricity. This is possible only during the daytime when sunlight is available. An interesting and complementary prospect is storing the absorbed solar energy by converting it into a different form of energy, such as chemical energy, which can then be transformed into electrical energy when sunlight is not available during the night-time.To realise this prospect, Assistant Professor Dr Baswanth Oruganti from the Department of Chemistry has designed a molecule that can absorb solar energy and convert it into the chemical energy of the bonds. His paper titled Modulating the Photocyclization Reactivity of Diarylethenes through Changes in the Excited-State Aromaticity of the π-Linker has been published in the Journal of Organic Chemistry, on Cover Page, with an impact factor of 4.2. He is both the first author as well as the corresponding author of the article. For this project, he has collaborated with Prof Bo Durbeej, Division of Theoretical Chemistry, Department of Physics, Chemistry, and Biology (IFM), Linköping University, Sweden.
Abstract
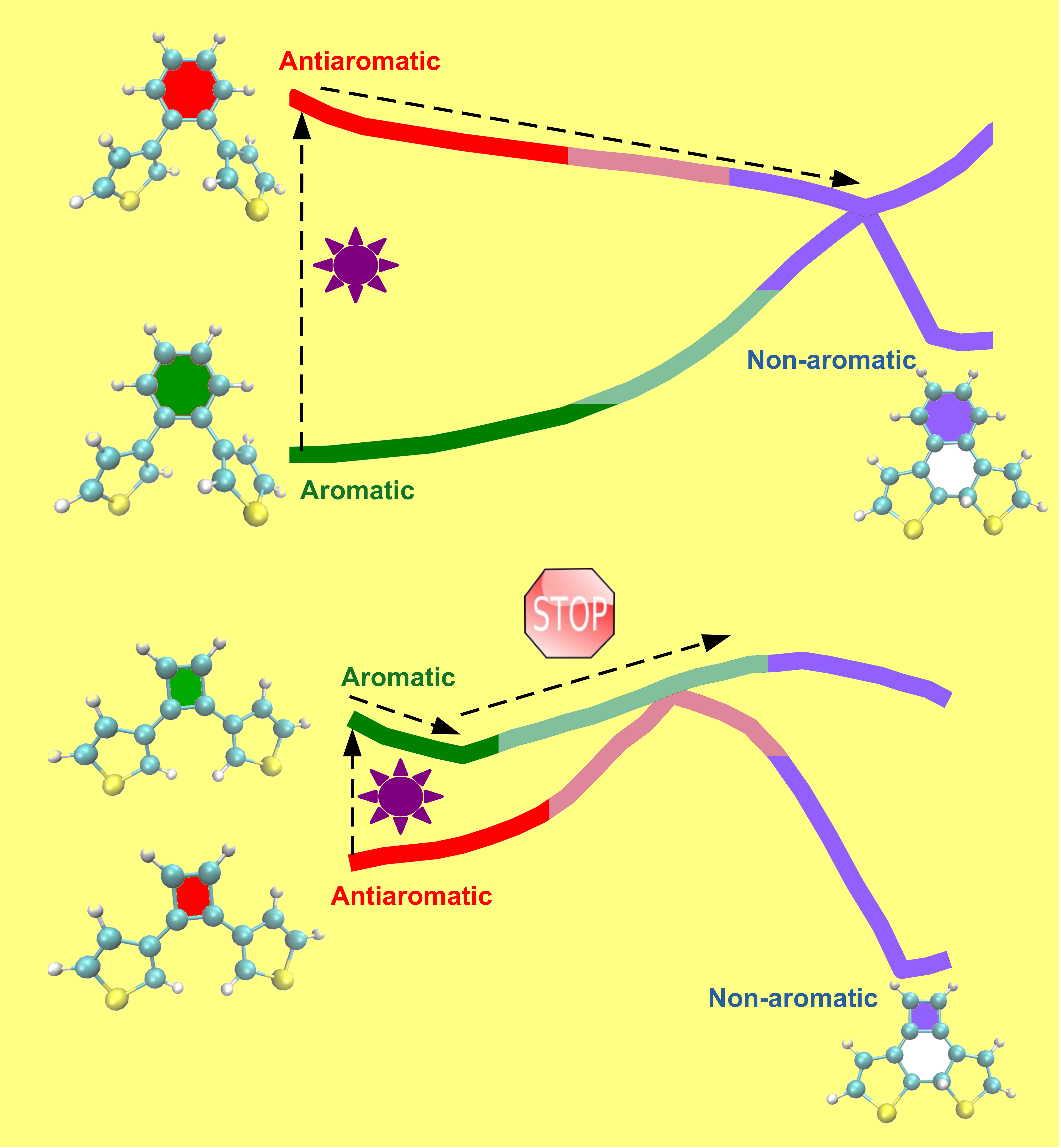
In recent years, the concept of excited-state aromaticity and its applications in photophysics and photochemistry has attracted considerable research interest. Our study uses quantum chemical calculations to systematically investigate if the photocyclization reactivity of diarylethene switches can be controlled by the excited-state aromaticity of the ethene bridge. Indeed, we demonstrate that these switches can be transformed from being highly reactive to completely non-reactive by changing the excited-state character of the bridge from anti-aromatic to aromatic.
Generally, molecules tend to move from a high-energy state to a low-energy state, as the lowering of energy increases the stability of the molecule and makes it chemically less reactive. In contrast, the present study shows that it is possible to chemically transform a molecule from a low-energy (aromatic) state to a high-energy (non-aromatic) state by absorption of light. This reaction occurs via a high-energy (anti-aromatic) electronically excited state of the molecule induced by light and has potential applications for storing solar energy in the form of chemical energy.
One challenge in the design of molecular solar energy storage systems, such as the diarylbenzene designed in the study, is that it is difficult to store solar energy for a longer period due to the instability of the newly formed chemical bonds at room temperature. To store solar energy for a longer period, one needs to compromise on the amount of energy stored in the bonds. In this regard, in the future, researchers are planning to optimise their molecular design by finding the right balance between the amount of solar energy stored and the time period for which it can be stored.
Continue reading →

