Recent News
- Faculty Development Programme on Recent advancements in Materials Chemistry September 6, 2022
 Material Chemistry is rapidly emerging as a critical component of contemporary science. Due to its interdisciplinary nature, the field requires input from all diverse branches of Chemistry. The Department of Chemistry is organising a Faculty Development Programme on Recent advancements in Materials Chemistry. Renowned academicians Prof Srinivas Hotha, IISER Pune, and Prof Chilla Malla Reddy, IISER Kolkata, will be the programme’s keynote speakers.
Material Chemistry is rapidly emerging as a critical component of contemporary science. Due to its interdisciplinary nature, the field requires input from all diverse branches of Chemistry. The Department of Chemistry is organising a Faculty Development Programme on Recent advancements in Materials Chemistry. Renowned academicians Prof Srinivas Hotha, IISER Pune, and Prof Chilla Malla Reddy, IISER Kolkata, will be the programme’s keynote speakers.Prof Srinivas Hotha will deliver a talk on Discovery and Development of Gold-Catalyzed Glycosylation for the Synthesis of Oligosaccharides, and Prof Chilla Malla Reddy will handle a session on Adaptive Soft Molecular Crystals: From Bending to Self-healing Assistant Professor Dr Nimai Mishra and DST – Ramanujan Fellowship Faculty Dr Satheesh Ellipilli are the convenors of the event.
Date: September 7, 2022
Time: 2 PM to 5 PM
About the speakers
Prof Srinivas Hotha is the Dean of Planning and Communications at IISER Pune. He is an expert faculty in Chemistry and Glycochemical Biology. He has more than 25 years of experience in the field of teaching and research. He completed his PhD at Osmania University, Hyderabad.
Prof Chilla Malla Reddy is from the Department of Chemical Sciences of IISER Kolkata. He did his PhD in Supramolecular Chemistry and Crystal engineering from the University of Hyderabad. He continued his research as a post-doctoral fellow at Karlsruhe Institute of Technology, Germany. He has been awarded the prestigious Swarna Jayanti fellowship by the Department of science and technology, Government of India.
Continue reading → - PhD scholars attended the INUP-i2i Familiarisation Workshop at IIT Kharagpur August 18, 2022
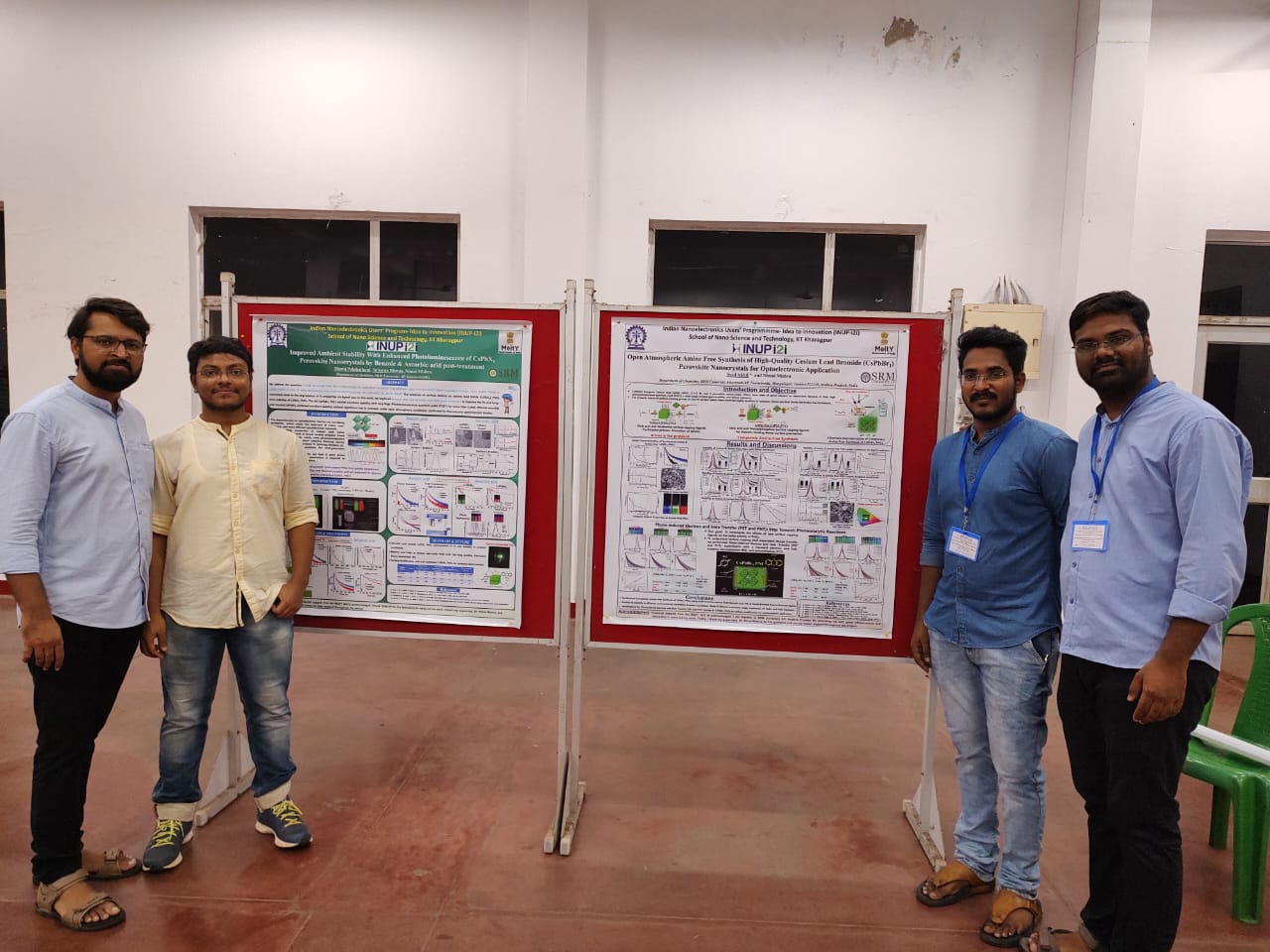 The Ministry of Electronics and Information Technology (MeitY) established the Indian Nanoelectronics User’s Programme (INUP) about a decade ago with the intention of improving skilled manpower in the areas of micro and nanoelectronics. This has laid the necessary foundation for the next step of the programme, INUP-i2i. It is a matter of pride that four PhD students from the Department of Chemistry attended the INUP-i2i Familiarisation Workshop on Nanofabrication and characterisations held from August 10 to 12, 2022, at IIT Kharagpur. Mr Syed Akhil, Mr Rahul SIngh, Mr Manoj Palabathuni, and Mr Subarna Biswas are the scholars who have grabbed this incredible opportunity.
The Ministry of Electronics and Information Technology (MeitY) established the Indian Nanoelectronics User’s Programme (INUP) about a decade ago with the intention of improving skilled manpower in the areas of micro and nanoelectronics. This has laid the necessary foundation for the next step of the programme, INUP-i2i. It is a matter of pride that four PhD students from the Department of Chemistry attended the INUP-i2i Familiarisation Workshop on Nanofabrication and characterisations held from August 10 to 12, 2022, at IIT Kharagpur. Mr Syed Akhil, Mr Rahul SIngh, Mr Manoj Palabathuni, and Mr Subarna Biswas are the scholars who have grabbed this incredible opportunity.Indian Nanoelectronics User’s Programme- Idea to Innovation (INUP-i2i) is developed to facilitate and support the generation of expertise in Nanoelectronics through participation and utilisation of the facilities at Nano-centres at IISc Bangalore, IIT Bombay, IIT Delhi, IIT Kharagpur, IIT Madras, and IIT Guwahati.
INUP will provide easy access to state-of-the-art nanofabrication and characterisation facilities to researchers, thereby creating a critical mass of hands-on experimental researchers across the country. This workshop is being organised both for familiarisation and interaction of the participants with faculty members of IITKGP. INUP has provided the accommodation and food for these shortlisted students. At the end of the workshop, they presented a poster as well.
Continue reading → - Charge transfer in photoexcited cesium lead halide perovskite nanocrystals August 8, 2022
The Department of Chemistry is glad to announce that Assistant Professor Dr Nimai Mishra and his research group Manoj Palabathuni, Syed Akhil, and Rahul Singh have published an article titled “Charge Transfer in Photoexcited Cesium Lead Halide Perovskite Nanocrystals: Review of Materials and Applications” in the Q1 journal “ACS Applied Nano Materials ” published by The American Chemical Society. The journal has an Impact Factor of 6.14.
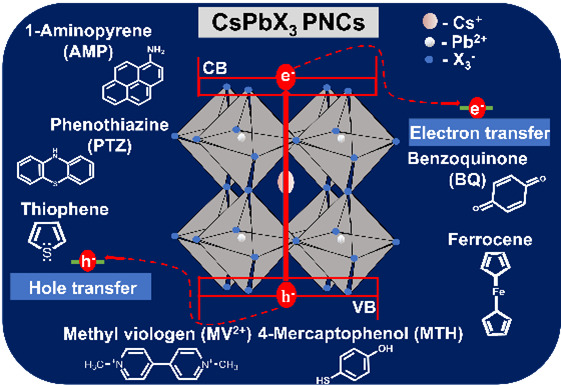
Cesium Lead Halide (CsPbX3) perovskite nanocrystals (PNCs) have attracted significant views from researchers due to their essential optoelectronic properties, especially long charge carrier transfer, high efficiency in visible light absorption, and long excited states lifetime, etc. Because of these properties, these materials exhibit outstanding charge transfer and charge separation, which enables them for solar cell applications. Recently, cesium lead halide perovskites have emerged as photocatalysts. In photovoltaics or photocatalysis, upon photoexcitation, the exciton dissociates, and the electron/hole is transmitted from the conduction/valance bands to the electron/hole acceptors. Therefore, it is essential to understand how the charge transfer occurs at the PNCs interface, which can help the researcher maximize the output in solar cells and photocatalytic efficiency.
In this article, Dr Mishra’s research group has outlined different charge transfer dynamics based on critical factors and discussed their optoelectronic properties. Electron/hole transfer dynamics are the most concerning characteristic; thus, they reviewed the relevant literature that reported efficient electron/hole transfer performance. In the end, they highlighted the recent development of the use of perovskite nanocrystal as photocatalyst in organic synthesis.
Continue reading → - Optimised copper nanoclusters for bio imaging applications July 15, 2022
Inspite of being a plentiful and inexpensive metal, the use of copper nanoclusters is limited in bio-medical research because of their toxicity and low stability due to its easily oxidizable nature. It also has a low quantum yield. The interdisciplinary publication of the researchers at SRM University-AP successfully addressed these constraints, resulting in strong fluorescence, superior colloidal stability, and non-toxicity of copper nanoclusters for bio imaging applications. The research was a collective work of Dr Manjunatha Thondamal from the Department of Biological Sciences, Dr Mahesh Kumar Ravva and Dr Sabyasachi Chakrabortty from the Department of Chemistry along with their PhD scholars; Mr Kumar Babu Busi, Ms Kotha Jyothi, Ms Sheik Haseena, Ms Shamili Bandaru and Ms Jyothi Priyanka Ghantasala.
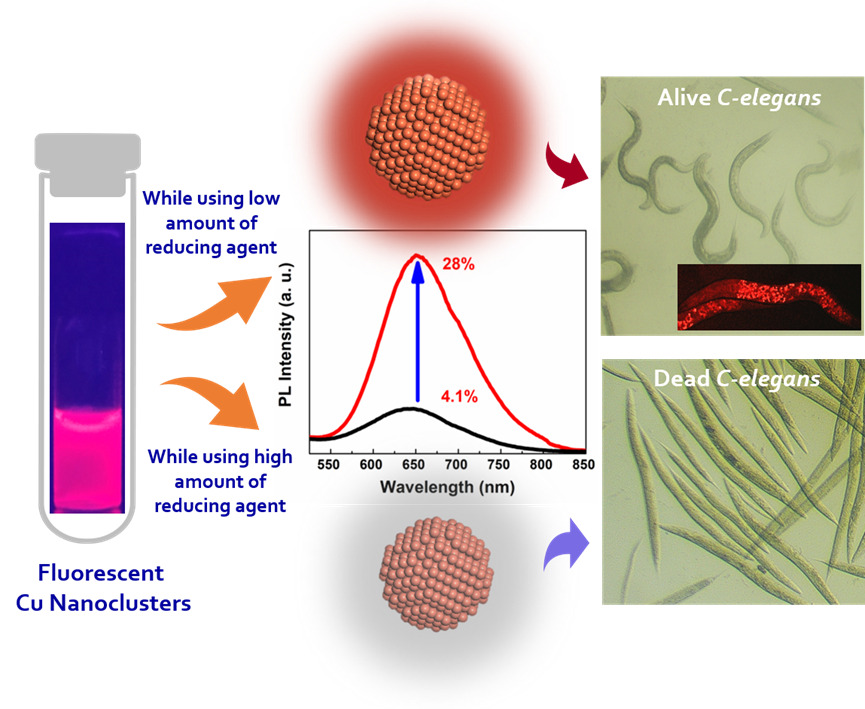
The article titled ‘“Engineering colloidally stable, highly fluorescent and nontoxic Cu nanoclusters via reaction parameter optimization” was featured in the prestigious Q1 journal RSC Advances (IF: 4.036), published by the ‘Royal Society of Chemistry’. They successfully prepared the protein stabilised copper nanoclusters inside the aqueous medium with exceptional optical properties. To the best of their knowledge, the reported colloidal stability and quantum yield of their as-synthesized Cu NCs are the highest reported in the literature, where the emission wavelength is in the red region. Also, optimised copper nanoclusters showed excellent biocompatibility towards solid cancer cell lines and C. elegans as in vitro and in vivo environments. Thus, these red colour luminescent copper nanoclusters were becoming a suitable fluorescent probe for deep tissue penetration, photodynamic, photothermal and diagnostic applications.
Abstract of the Research
Metal Nanoclusters (NCs) composed of the least number of atoms (few to tens) became very attractive for their emerging properties owing to their ultrasmall size. Preparing copper nanoclusters (Cu NCs) in an aqueous medium with high emission properties, strong colloidal stability, and low toxicity has been a long-standing challenge. Although they are earth-abundant and inexpensive, they are comparatively less explored due to their limitations such as ease of surface oxidation, poor colloidal stability, and high toxicity. To overcome these constraints, we established a facile synthetic route by optimizing the reaction parameters, especially altering the effective concentration of the reducing agent to influence their optical characteristics. The improvement of photoluminescence intensity and superior colloidal stability was modelled from a theoretical standpoint. Moreover, the as-synthesized Cu NCs showed a significant reduction of toxicity in both in vitro and in vivo models. The possibilities of using such Cu NCs as a diagnostic probe towards C. elegans were explored. Also, the extension of this approach towards improving the photoluminescence intensity of the Cu NCs on other ligand systems was demonstrated.
Continue reading →

