Recent News
- Green hydrogen to combat global warming May 26, 2022
‘Energy Conversion & Management’ is a journal that belongs to the top 2% of the “Renewable Energy, Sustainability, and the Environment” subject category. Publishing a paper with an impact factor of 9.7 in such a journal is a considerable achievement. Assistant Professors Dr Sabyasachi Chakrabortty and Dr Mahesh Kumar Ravva and their PhD scholar Ms Mounika Sai Ambati from the Department of Chemistry have accomplished this by publishing a paper titled Photovoltaic/Photo-Electrocatalysis Integration for Green Hydrogen: A review in this Q1 journal.
Abstract of the research
Continue reading →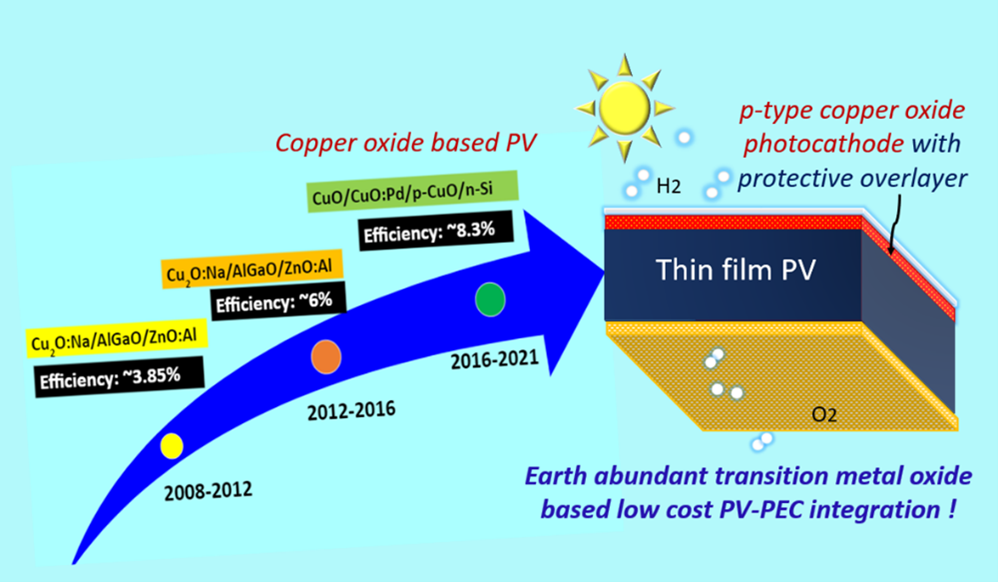 Solar light-driven hydrogen generation via water splitting is essential to combat global warming and CO2 emission. The production of hydrogen from fossil fuels produces massive amounts of CO2. Developing a sustainable and eco-friendly approach to hydrogen production is the need of the hour. Photoelectrochemical water splitting is a clean way to produce hydrogen by using water. The hydrogen generated through water splitting is referred to as Green Hydrogen. Photoelectrochemical water splitting uses metal oxides as photocathode/anode. The challenges that occur here are stability, low efficiency, and large-scale development (reusable electrodes are essential). Hence, the primary goal is to demonstrate photoelectrodes using different metal oxides by in-situ doping of different metals to detect the challenges.
Solar light-driven hydrogen generation via water splitting is essential to combat global warming and CO2 emission. The production of hydrogen from fossil fuels produces massive amounts of CO2. Developing a sustainable and eco-friendly approach to hydrogen production is the need of the hour. Photoelectrochemical water splitting is a clean way to produce hydrogen by using water. The hydrogen generated through water splitting is referred to as Green Hydrogen. Photoelectrochemical water splitting uses metal oxides as photocathode/anode. The challenges that occur here are stability, low efficiency, and large-scale development (reusable electrodes are essential). Hence, the primary goal is to demonstrate photoelectrodes using different metal oxides by in-situ doping of different metals to detect the challenges. - Inspiring the world with quality research facilities May 7, 2022
 SRM AP is known for its resources and facilities for pioneering research with the support of global leaders and SME’s while sticking to compliance and international regulations. Obtaining research excellence in every field of study has been a mission of the university. Recently, 50 MSc students from the Department of Chemistry, KBN College, Vijayawada, visited our university to explore the analytical and research facilities available here.
SRM AP is known for its resources and facilities for pioneering research with the support of global leaders and SME’s while sticking to compliance and international regulations. Obtaining research excellence in every field of study has been a mission of the university. Recently, 50 MSc students from the Department of Chemistry, KBN College, Vijayawada, visited our university to explore the analytical and research facilities available here. The research areas handled by the Department of Chemistry of SRM AP include the disciplines of chemical sciences, ranging from organic, inorganic, and physical, to theoretical or computational chemistry. The department’s highly disciplinary and collaborative environment is indeed inspiring, and it continues to grab attention. The strong interactions of the university with other premier institutions across India and around the world refine the quality of analytical and research facilities available here. The students from KBN college eagerly interacted with the faculty members and research scholars. Dr Mahesh Kumar Ravva and Dr Rajapandiyan, Faculty members, coordinated the visit.
Continue reading → - Presenting our first doctorate holder: Dr Vasavi Dutt April 21, 2022
 The university revels in its monumental achievement of bringing out the maiden doctorate degree holder, Dr Vasavi Dutt, within four years of its inception. Dr Vasavi Dutt enrolled as a PhD scholar in the Department of Chemistry, under the supervision of Dr Nimai Mishra, Assistant Professor, in 2018. She received the academic honour for her research thesis titled “Improvement of Photoluminescence and Achieving the Stabilization of Cesium Lead Halide Perovskite Nanocrystals for Light-emitting Applications”. Dr Vasavi has been an extremely diligent student and she mustered up immense courage to bring her research to closure even during the testing times of the pandemic.
The university revels in its monumental achievement of bringing out the maiden doctorate degree holder, Dr Vasavi Dutt, within four years of its inception. Dr Vasavi Dutt enrolled as a PhD scholar in the Department of Chemistry, under the supervision of Dr Nimai Mishra, Assistant Professor, in 2018. She received the academic honour for her research thesis titled “Improvement of Photoluminescence and Achieving the Stabilization of Cesium Lead Halide Perovskite Nanocrystals for Light-emitting Applications”. Dr Vasavi has been an extremely diligent student and she mustered up immense courage to bring her research to closure even during the testing times of the pandemic.In the words of Dr Nimai Mishra, “It was a great privilege for me to supervise Ms Vasavi, (correct me Dr Vasavi now) as my first PhD student. She joined my research lab in July 2018 when there was no lab at all, and we started our work at Chemistry BTech Lab”. Dr Mishra was gleaming with pride as he spoke more about his scholar, “During these three and a half years, I had relentless scientific discussions with Vasavi which enriched both of us. Her attitude towards research was remarkable, whenever I gave her a research problem, she used to come up with a detailed outline of how to go ahead with the project”. He also praised her for all her accomplishments which include the publication of 13 research papers, filing of 3 patents and winning the best poster in national & internal conferences.
Dr Vasavi also shared her happiness for having received the mentorship of Dr Mishra, “Working in Dr Nimai Mishra’s lab was a great experience. I had the opportunity to engage and initiate multiple research topics and collaborations. He has always encouraged me to explore new fields to broaden perspectives and bring together new ideas”. She also expressed her gratitude to him for being a welcoming and approachable mentor. “I’m eternally thankful to Dr Mishra for his friendship, empathy, and moreover, for his great sense of humour”. She currently resides in the US with her family. Now that she has successfully completed her PhD, soon she would start looking for a job or rather pursue a post-doctoral fellowship in America.
Dr Vasavi was out of words to thank the university for facilitating and bringing the best in technology and infrastructure for advanced research. “I can never thank my university enough for extending a hospitable environment and nutritious food for all the doctorate students”, she further mentioned. The university serves as a promised land for thousands of research aspirants like her to head towards their dream of making unfeigned contributions to academia.
- Do you think CSIR-JRF is a tough nut to crack? Persistence is the key April 21, 2022

“Believe you can and you’re halfway there.” – Theodore Roosevelt
Jesni M Jacob, currently doing research under Dr Mahesh Kumar Ravva narrates her journey to achieving CSIR-JRF All India Rank of 65 through persistent efforts.
I’m working in the field of computational chemistry on designing and developing organic molecules for OLED applications. Securing an AIR of 65 in the CSIR JRF in Chemical Science June 2021 exam is a dream come true moment for me.
In 2019, I completed my post-graduate studies at Madras Christian College, Chennai. The four-year-long journey from zero to JRF AIR 65 was of hard work, patience, sleepless nights, sacrifices and even frustrated moments. It was challenging to remain motivated after multiple unsuccessful attempts. But I wasn’t ready to give up hope. I believed in myself and dreamed big with faith in God Almighty.
My previous attempts didn’t provide me with any hope of continuing my preparation because my marks were consistently far below the cutoffs. That made me realise one thing: without coaching and ample guidance, qualifying for CSIR JRF is a toiling task for an average student. But I learned that with strong passion, proper dedication, and right strategies of do’s and don’ts, any aspiring student can pass the exam with flying colours.
After each attempt, I learned from my mistakes and tried to optimise my strategies. One should never try to cover the entire syllabus and be bothered about it. I analysed the unit-wise weightage and narrowed it down to a few important topics that I found exciting and comfortable.

- Choose topics carefully and focus solely on mastering them.
- Try to stick to and rely on reliable standard textbooks as much as possible.
- The SRMAP library provided me with excellent access to a wide range of standard texts.
The JRF aspirants should try to solve previous years’ questions from standard exams (CSIR, GATE, IISc, etc.) and note new concepts or approaches every day. Enjoy and prepare short notes with a lot of scribbling and highlighting in various colours. Notes should be concise and simple to revise later. But don’t spend too much time making notes.
I made time for exam preparation along with my work and research activities. I’m grateful to my family, teachers, and especially my guide- Dr Mahesh Kumar Ravva, for their constant support and encouragement. He gave me a safe space to express my desire to ace the exam and my anxieties about it. Dr Mahesh always listened to my concerns and helped me to gain clarity on my thoughts. He always encouraged me to dream big and shared his perspectives and lessons from his life experiences. He is a great mentor, motivator, and teacher to me.
Continue reading →












