Prof Ranjit Thapa and his PhD scholar, Mr Samadhan Kapse from the Department of Physics have reported their euphoric achievement of discovering an economically viable electrocatalyst for effective green urea synthesis. The paper “Selective Electrocatalytic Co-reduction of N2 and CO2 on Copper Phthalocyanine for Green Urea Production” has been published in the highly prestigious Nature indexed journal, ‘Advanced Functional Materials’, having an Impact Factor of 18.81. It was published in collaboration with Jit Mukherjee, and Uttam Kumar Ghorai, from the Department of Industrial Chemistry & Applied Chemistry, Swami Vivekananda Research Centre.
With global annual production of 100 million tons, urea is one of the important nitrogen sources for the fertilizer industry. Industrial urea is synthesized by the following two consecutive steps. First, the reaction of nitrogen and hydrogen (N2 + H2 → NH3) by the Haber-Bosch process at high temperature and pressure (350–550°C, 150–350 bar); followed by the reaction of NH3 and CO2 [NH3 + CO2 → CO(NH2)2] under mild reaction conditions (170–200°C and 200–250 bar). The sequential reactions are carried out for several cycles to increase the conversion efficiency. For the first step, fixation of N2 is an energy as well as a capital intensive process due to difficulty in cleaving the N≡N bond. Extensive research works have been reported on electrochemical N2 fixation to NH3 in water medium under ambient conditions. In this electrochemical method, isolation of NH3 gas with high purity from electrolyte solution is troublesome. In the second step, CO2 fixation on the substrate and its separation is one of the major challenging tasks for the further reaction with NH3 to end up in urea formation. Overall, the two-step process for large scale production of urea consumes high energy and produces greenhouse gases for the environment.
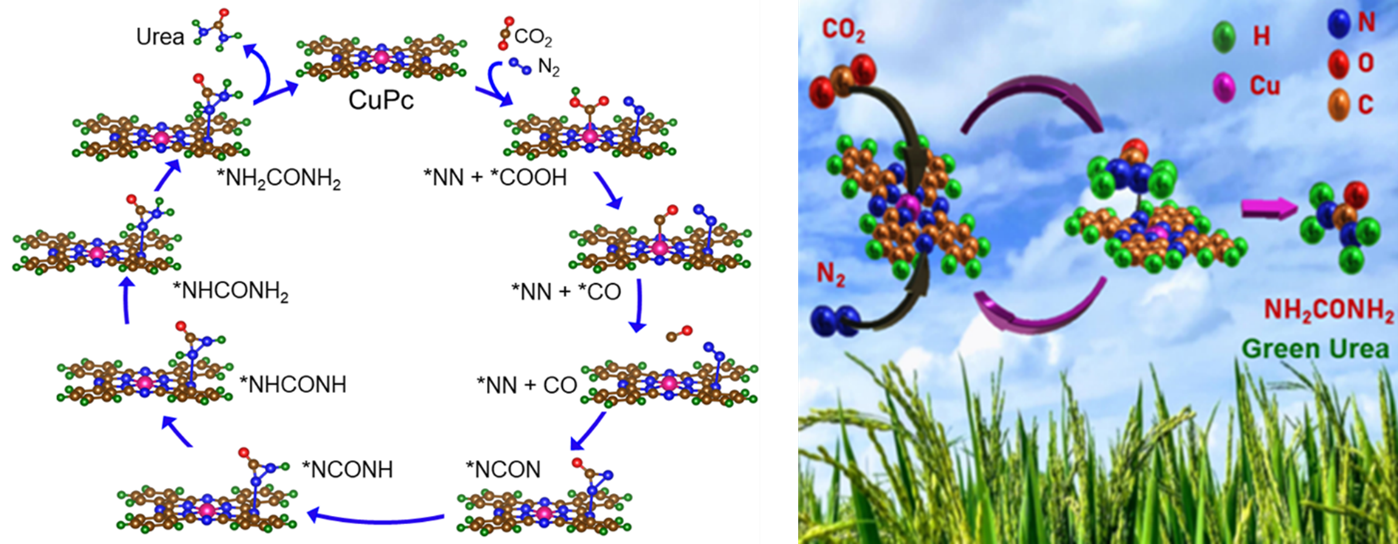
The research team reported copper-phthalocyanine nanotubes (CuPc NTs) having multiple active sites as an efficient electrocatalyst which exhibits a tremendous yield of urea with good durability and long-term stability. DFT calculation predicts that Pyridinic–N1 in CuPc is responsible for N2 reduction and the metal centre plays an important role for CO2 reduction. This study not only provides us with the co-reduction of N2 and CO2 gases using cost-effective CuPc NTs catalyst but also opens a new pathway to the rational design of other transitional metal-based electrocatalysts having multiple active sites for N2 and CO2 gas fixation applications.
This electrochemical method of urea synthesis by the co-reduction of N2 and CO2 [N2 + CO2 + 6H+ + 6e– → CO(NH2)2 + H2O] using an efficient electrocatalyst in a water medium under ambient conditions would be an alternative way in the upcoming days. All the strategies using alloys and heterostructure for urea synthesis forming C–N bond by the co–reduction of N2 and CO2 have not reached the benchmark in terms of urea yield rate and FE for practical applications. To achieve a high urea yield and FE, various factors are to be considered in this work.
Abstract of the Research
Green synthesis of urea under ambient conditions by electrochemical co-reduction of N2 and CO2 gases using effective electrocatalyst essentially pushes the conventional two steps (N2 + H2 = NH3 & NH3 + CO2 = CO (NH2)2) industrial process at high temperature and high pressure, to the brink. The single-step electrochemical green urea synthesis process has hit a roadblock due to the lack of an efficient and economically viable electrocatalyst with multiple active sites for dual reduction of N2 and CO2 gas molecules to urea. Herein, the research reports copper-phthalocyanine nanotubes (CuPc NTs) having multiple active sites (such as metal centre, Pyrrolic-N3, Pyrrolic-N2, and Pyridinic-N1) as an efficient electrocatalyst which exhibits urea yield of 143.47 µg h-1 mg-1cat and FE of 12.99% at –0.6 V vs RHE by co-reduction of N2 and CO2. Theoretical calculation suggests that Pyridinic-N1 and Cu centres are responsible to form C–N bonds for urea by co-reduction of N2 to NN* and CO2 to *CO respectively. This study provides new mechanistic insight into the successful electro-reduction of dual gases (N2 and CO2) in a single molecule as well as the rational design of an efficient noble metal-free electrocatalyst for the synthesis of green urea.

