Renowned physicists deliver talks at the “One-Day National Symposium on High Energy Physics.”
 The Department of Physics, SRM University-AP organized a “One-day National Symposium on High Energy Physics” on Saturday, May 1, 2021. The session was held through online mode in the presence of honourable leaders of the university, faculty members, and attendees from various fields of interest. Prof V S Rao, the Vice-Chancellor, SRM University-AP, welcomed the gathering with a brief overview of the university’s inception and quick progress in the field of research.
The Department of Physics, SRM University-AP organized a “One-day National Symposium on High Energy Physics” on Saturday, May 1, 2021. The session was held through online mode in the presence of honourable leaders of the university, faculty members, and attendees from various fields of interest. Prof V S Rao, the Vice-Chancellor, SRM University-AP, welcomed the gathering with a brief overview of the university’s inception and quick progress in the field of research.
Three talks at the pedagogical level were organised for a wider audience, especially for the students of basic sciences and engineering streams. The first speaker, a Padma Shri awardee, Prof Rohini Godbole, who is a theoretical particle physicist at Indian Institute of Science (IISc), Bengaluru expounded on “Status of Particle Physics: in light of Nobel Prizes of 2013 and 2015”. She talked about the Higgs Boson’s theoretical postulate, for which the Nobel Prize in Physics was granted in 2013. According to studies, it was the final missing element in the Standard Model’s periodic table (SM). Her paper incorporated the experimental finding of the Neutrino Oscillation, as well as many compelling pieces of evidence that led to the 2015 Nobel Prize.
In the afternoon session, the talks were on the burgeoning field of Dark Matter physics and Gravitational-wave astronomy. Prof Basudeb Dasgupta from Tata Institute of Fundamental Research (TIFR), Mumbai apprised the participants on “The Mystery of Invisible Mass”. The deep understanding and expertise of Prof Dasgupta in the interfaces of particle physics, astrophysics, and cosmology with a particular emphasis on dark matter and neutrino physics as a theoretical physicist at the Tata Institue of Fundamental Research (TIFR) enlightened the audience. He stated that the human beings are able to see only 20% of the universe’s celestial objects; the remaining 80% are unseen and are referred to as “dark matter.” In his words, “Billions of years after the Big Bang, all we can see is the cloud’s surface, where the light is scattered.” Prof Basudeb, is a frequent speaker at prestigious international and national conferences and is a youth icon for aspiring physicists and scientists.
The final lecture of the day was delivered by Prof Bala Iyer from International Centre for Theoretical Sciences (ICTS), Bengaluru on “The Detection of Gravitational Waves and the Dawn of Multi-messenger Astronomy” at 4.00 pm. Prof Iyer is currently the Simons Visiting Professor at ICTS-TIFR Bangalore and co-PI of the LIGO-India Scientific Collaboration. He coaches and guides young minds interested in astrophysics, cosmology, and fundamental physics. Prof Iyer did a presentation on the discovery of gravitational waves from a binary black hole in 2015, which was a watershed moment and necessitated the launch of a new multi-messenger astronomy with the potential to have a significant impact on astrophysics. “Any relativistic theory of gravity must be consistent with the special relativity principle. Gravity’s effect cannot travel faster than the speed of light. If an item’s gravitational field changes, the changes propagate over space and take a certain amount of time to reach the object “, he added.
The symposium that aimed to discuss the current status of exciting research topics of High Energy Physics concluded with a Q&A session. This has proved that the faculty members and participants were highly inspired and motivated after attending the symposium and listening to the scholars.
Pre-Event Release: https://srmap.edu.in/events/national-symposium-on-high-energy-physics-2021/
- Published in News, Physics News
Department of Physics set to work on bilateral project with IIT Bhilai
 Dr. Satyajit Gupta (PI), IIT Bhilai, and Dr Sabyasachi Mukhopadhyay (Co-PI), Department of Physics, SRM University-AP has signed a Memorandum of Understanding for the DST (Indo-Israel Joint Research Co-operation-IIJRC) sponsored project entitled “A HALIDE PEROVSKITE BASED PHOTOANODE FOR OXYGEN EVOLUTION REACTION USING A MOLECULAR DIODE IN A HYBRID NANOMETER SCALE PROTECTION LAYER”, Sanction Order NO. – DST/INT/ISR/P-28/2020(G). The project is a bilateral project and Foreign PI is Dr Eran Edri, Department of Chemical Engineering, Ben-Gurion University of the Negev, Israel. This MoU will help Dr Mukhopadhyay to utilize the fund under this project as co-PI, and the facility of IIT Bhilai to complete the objective of the project.
Dr. Satyajit Gupta (PI), IIT Bhilai, and Dr Sabyasachi Mukhopadhyay (Co-PI), Department of Physics, SRM University-AP has signed a Memorandum of Understanding for the DST (Indo-Israel Joint Research Co-operation-IIJRC) sponsored project entitled “A HALIDE PEROVSKITE BASED PHOTOANODE FOR OXYGEN EVOLUTION REACTION USING A MOLECULAR DIODE IN A HYBRID NANOMETER SCALE PROTECTION LAYER”, Sanction Order NO. – DST/INT/ISR/P-28/2020(G). The project is a bilateral project and Foreign PI is Dr Eran Edri, Department of Chemical Engineering, Ben-Gurion University of the Negev, Israel. This MoU will help Dr Mukhopadhyay to utilize the fund under this project as co-PI, and the facility of IIT Bhilai to complete the objective of the project.
The Objectives of the MoU are to promote effective application of resources through Indo-Israel Joint Research Co-operation-(IIJRC) sponsored project, promote mentorship and research guidance, and cooperate in educational/research areas of mutual interest. It also aims to promote international collaborations through International travel of Party, hosting International delegates, and through a student exchange programme between Indian Institute/Universities and Ben-Gurion University of the Negev, Israel.
The MoU will provide a platform to share and exchange Best Practices, and facilitate exchange programmes for students. Dr Satyajt Gupta and Dr Sabyasachi Mukhopadhyay will provide training and development for students working under this joint project.
- Published in Departmental News, News, Physics News
Physics student files patent
 Ms Sreelekha Bhuvaneswari, a BSc physics final year student, in SRM University AP, Andhra Pradesh, filed a patent for her work titled “A fibre material with moisture retention capacity with thermal tolerance and a method for manufacture” under the guidance of Dr Sabyasachi Mukhopadhyay, Assistant Professor, Department of Physics, SRM University-AP.
Ms Sreelekha Bhuvaneswari, a BSc physics final year student, in SRM University AP, Andhra Pradesh, filed a patent for her work titled “A fibre material with moisture retention capacity with thermal tolerance and a method for manufacture” under the guidance of Dr Sabyasachi Mukhopadhyay, Assistant Professor, Department of Physics, SRM University-AP.
The project, with the patent application number 202141023375, develops a methodology to design a fabric cloth that would replace the use of air conditioners. This cloth design is inspired by Saharan silver ants which regulate their body temperatures in the scorching desert heat and also from the cooling properties of clay. This research would significantly scale down the usage of AC and other cooling devices in warm places, thus reducing the use of electricity and emission of greenhouse gases to the environment. As this cloth would be environment friendly with long durability and cost-efficiency, Sreelekha hopes that this research would bridge the socioeconomic divide of haves and have-nots between communities.
“I am grateful to Dr Sabyasachi sir for his constant help and guidance along the way. There were several failed models, but he believed in the concept and that inspired me to go forward with the project,” said Ms Bhuvaneswari. “The facilities at the University made the process seamless; once the proposal was made, the procedure was automated. I thank the officials of SRM University-AP for believing in my proposal and helping me get through the procedures smoothly. If it were not for the facilities available at my university, I could not have finished the design,” She added.
- Published in News, Physics News, Research News, Students Achievements
Controlled loading of MoS2 on hierarchical porous TiO2 for enhanced photocatalytic hydrogen evolution
 Ever since the breakthrough research on H2 photogeneration from water using TiO2 under UV-light irradiation, an enormous amount of research has been conducted on photochemical H2 evolution using different semiconductor-based photocatalysts. Consequently, a research paper titled “Controlled Loading of MoS2 on Hierarchical Porous TiO2 for Enhanced Photocatalytic Hydrogen Evolution” has been published by Prof Ranjit Thapa, Professor of Physics, SRM University – AP, as a co-author, in The Journal of Physical Chemistry C, having an Impact Factor of 4.189.
Ever since the breakthrough research on H2 photogeneration from water using TiO2 under UV-light irradiation, an enormous amount of research has been conducted on photochemical H2 evolution using different semiconductor-based photocatalysts. Consequently, a research paper titled “Controlled Loading of MoS2 on Hierarchical Porous TiO2 for Enhanced Photocatalytic Hydrogen Evolution” has been published by Prof Ranjit Thapa, Professor of Physics, SRM University – AP, as a co-author, in The Journal of Physical Chemistry C, having an Impact Factor of 4.189.
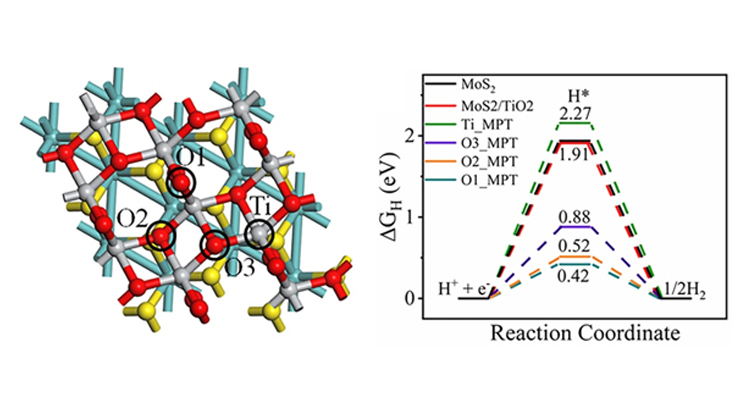
In this work, Prof Thapa describes three important factors for helping in the generation of hydrogen using proposed MoS2/TiO2 catalyst, (i) TiO2 for effective charge transfer, (ii) MoS2 for plasmon induction (iii) large surface area and active sites. It was shown that hierarchical porous TiO2 can be interfaced successfully with marigold-flower-like MoS2 flakes with intriguing photophysical properties, viz., visible-light response, controlled electron−hole recombination, and sustainable H2 production over prolonged light irradiation due to the synergic effect of flowerlike MoS2 and the fibrous wormhole mesoporous channel of TiO2. Further, the researchers have used density functional theory (DFT) to identify the active sites and calculated the change in Gibbs free energy (ΔGH). “We have also studied the charge density difference to understand about electron transfer pathway. The change free energy of hydrogen adsorption (ΔGH*) is a good indicator to estimate the hydrogen evolution activity in the acidic medium. From the DFT study, it is clear that O sites of MPT heterostructure are more favourable for HER reactivity”, said Prof Ranjit Thappa.
Social implications of the research:
In the last few decades, with the decline in non-renewable resources and increasing environmental pollution, significant attention has been given to renewable and clean energy domains. Hydrogen is considered one of the most suitable energy carriers due to its higher energy density per unit mass in comparison to other chemical fuels. In recent times, photocatalytic fission (Photocatalysis is a process in which light energy is used to drive pairs of chemical reactions. Through the absorption of light, an excited electron/hole pair is produced) of water has been considered an attractive solution for solar to chemical H2 energy conversion. Also, the process of water splitting is highly endothermic. Therefore, the development of an excellent, stable, efficient, and economical photocatalyst for ultrahigh H2 production efficiency is paramount to researchers.
This work is done in collaboration with the Department of Energy and Environmental Engineering, CSIR-Indian Institute of Chemical Technology, Hyderabad 500007, India.
Prof Ranjit Thapa is doing an investigation to find the possibility of hydrogen evolution reaction (HER) on multiple borophene analogues (α, β12, χ3) on all unique sites. Understanding the role of the coordination number of the boron atoms in the borophene analogues with the HER efficiency, and studying the pathways Volmer-Tafel (V-T) on each site to understand the completed HER process on borophene analogues are his future research projects. His research group is also interested to identify the role of sigma and pi-electron occupancy on the V-T pathway.
Read the full paper here: https://doi.org/10.1021/acs.jpcc.1c01922
- Published in Departmental News, News, Physics News, Research News
Cost-effective electro-catalyst for oxygen reduction reaction
 A research paper titled “Nitrogen doping derived bridging of Graphene and Carbon Nanotube composite for oxygen electroreduction” has been published by Prof Ranjit Thapa, Professor of Physics, SRM University – AP, as a co-author, in International Journal of Energy Research, having Impact Factor of 5.164.
A research paper titled “Nitrogen doping derived bridging of Graphene and Carbon Nanotube composite for oxygen electroreduction” has been published by Prof Ranjit Thapa, Professor of Physics, SRM University – AP, as a co-author, in International Journal of Energy Research, having Impact Factor of 5.164.
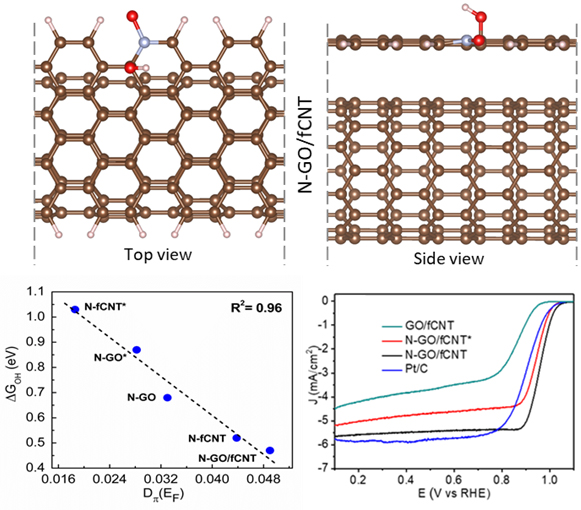
In this work, the research group investigated the origin of high catalytic activity of oxidic-N configuration in nitrogen-doped CNT and graphene heterostructure using density functional theory (DFT). We have plotted the free energy profile of the oxygen reduction reaction (ORR) to estimate the thermodynamic overpotential and catalytic performance of the respective active sites. The overpotential is related to the quantifying parameter ∆GOH (with R2 = 0.98) and the π electron density at the Fermi level, defined as an electronic descriptor, which is highly correlated with the ∆GOH with R2 value 0.96. For various N doped configurations, we correlated the ∆GOH values with π electron density at the Fermi level and found that the carbon site adjacent to the oxide-N configuration is a more prominent site for ORR. Further, we show that the oxidic-N configuration in the heterostructure of graphene and CNT is the ideal configuration, which gives a lower overpotential of 0.54 eV for ORR on adjacent carbon sites. Therefore, the charge transfer occurs from underneath CNT to graphene and increases the value of π electron density at the Fermi level which leads to the higher catalytic performance of the active site.
In the early 20th century, fuel cells were invented and their global impact has not reached up to its regular commercialization when compared with battery technology. The fuel cell device could be a powerful technique to generate electricity for large energy demand without greenhouse gas emissions. However, other major hurdles in the commercialization of fuel cell devices are the cost of platinum (as a catalyst), its poisoning and stability. Recently, carbon-based materials such as graphene, carbon nanotubes and activated carbon have been evolved as metal-free low-cost catalysts due to their (i) high abundance/yield (ii) reactivity towards oxygen just by introducing impurities like heteroatoms or other metals. However, identifying an efficient design principle to search optimal doping configurations in various carbon systems is a grand challenge for researchers.
This work is done in collaboration with Research Institute, SRM Institute of Science & Technology, Kattankulathur-603203, Chennai (India).
In future, the study aims to propose the effective design principle for various doped carbon systems as a catalyst to identify the optimal active sites and configurations for ORR. The role of π orbital in carbon systems such as graphene, graphene nanoribbons, carbon nanotube, etc is very important and can be a general electronic descriptor to define catalytic activity. Also, π electron descriptors and machine learning algorithms based combined approach can boost the search for ideal carbon catalyst for ORR with low DFT cost.
Read the full paper here: https://doi.org/10.1002/er.7179
- Published in Departmental News, News, Physics News, Research News
Prof U Ramamurty, renowned researcher from NTU Singapore, visits SRM University-AP
 An interactive session between Prof U Ramamurty, President Chair Professor, School of Mechanical & Aerospace Engineering at Nanyang Technological University (NTU), Singapore, and the faculty members of SRM University – AP, Andhra-Pradesh was held on Monday.
An interactive session between Prof U Ramamurty, President Chair Professor, School of Mechanical & Aerospace Engineering at Nanyang Technological University (NTU), Singapore, and the faculty members of SRM University – AP, Andhra-Pradesh was held on Monday.
During the discussion, Prof Ramamurty emphasized the importance of research collaboration between faculty members from different research areas and about utilizing expertise to achieve significant scientific output.
Dr Pardhasaradhi Maram from the Department of Chemistry, Dr Sabyasachi Mukhopadhyay from the Department of Physics, and Prof G S Vinod Kumar from the Department of Mechanical Engineering presented their detailed research areas that focus on storage devices, catalysts for value-added products, energy and sensing devices, novel metallic materials, additive manufacturing of metals and Bio-implants, and industry collaborative research work.
Prof Ramamurty said that he is glad to see that productive science is being done at SRM University-AP. “Given that the University has started only 4 years ago and been functioning amidst a pandemic for more than one and a half years, the progress in research is significant and very impressive. Interdisciplinary efforts between various departments in the University will give effective results”, he added.
Prof D Narayana Rao, Pro-Vice-Chancellor, SRM University – AP expressed his interest in establishing NTU – SRM joint Centre for Advanced Research in functional and structural materials at SRM University campus to Prof Ramamurty. The centre that Prof Rao envisions will provide an opportunity to synergize the expertise and resources of NTU, Singapore, and SRM University – AP to carry out front-line research in the areas of novel materials, self-healing materials and also additive manufacturing (3D Printing of metals and bio-implants).
- Published in Chemistry-news, Departmental News, Mechanical Engineering NEWS, News, Physics News, Research News
Third year CSE students innovate efficient plastic recycling technology
 Swikriti Khadke, Pragya Gupta, and Shanmukh Rachakunta from third-year Computer Science Engineering have published a research paper titled “Efficient Plastic Recycling and Remold Circular Economy using the Technology of Trust – Blockchain” along with their mentors from SRM University-AP Dr Jatindra Kumar Dash, Dr Goutam Kumar Dalapati and Dr Sabyasachi Chakrabortty in the peer-reviewed journal Sustainability.
Swikriti Khadke, Pragya Gupta, and Shanmukh Rachakunta from third-year Computer Science Engineering have published a research paper titled “Efficient Plastic Recycling and Remold Circular Economy using the Technology of Trust – Blockchain” along with their mentors from SRM University-AP Dr Jatindra Kumar Dash, Dr Goutam Kumar Dalapati and Dr Sabyasachi Chakrabortty in the peer-reviewed journal Sustainability.
Global plastic waste is increasing rapidly. The strategic management of plastic waste and recycling can preserve environmental species and associated costs. The utilization of plastic can be done by introducing Blockchain during plastic waste recycling. Automation for the segregation and collection of plastic waste can effectively establish a globally recognizable tool using Blockchain-based applications. Collection and sorting of plastic recycling are feasible by keeping track of plastic with unique codes or digital badges throughout the supply chain. Efficient recycling technology is essential to reduce plastic pollution. Many technologies have been employed to enhance plastic recycling. Among them, blockchain is promising for plastic recycling and circular economy (CE). Blockchain, a distributed ledger, consists of some ordered blocks which are unchangeable. This can be considered an exemplary way to push the transactions of their customers under the same blockchain technology. The research group used machine learning techniques to predict plastic generation globally so that they could see the impact it will make in the coming future. The students have used ARIMA – Auto-Regressive Integrated Moving Average for the study.
The potential idea is to utilize an approach wherein recyclers can keep track of generated waste as it moves through the various chains. A platform that works by tracking recycling activities across a local recycling supply chain on the Blockchain. When this will be publicly available, consumers can also use the ledger info to make more informed purchasing decisions. The Blockchain can be utilized to track individual items through the recycling supply chain by creating physical markers like QR codes.
The suggested Blockchain-based platform can be implemented in various nations with an autonomous waste collector and storage system. This process can be expanded to individual collectors and storage systems. The novel process will be created by incorporating a reward-based Blockchain scheme with the collaboration of global businesses and local waste collectors. The proposed model further allows the effective sharing of databases among various supply chains to create a CE.
Talking about the social implications of the research, the students firmly believe that the study will result in the introduction of new technology in the recycling industry and promote awareness about technology in rural areas. Developing a platform and implementing blockchain and other facilities will be the focus of these young innovative brains of SRM University-AP in the forthcoming days.
Read the full paper here: https://doi.org/10.3390/su13169142
- Published in Chemistry-news, Computer Science News, Departmental News, News, Physics News, Research News
BSc Physics student contributes as the First Author for a research paper
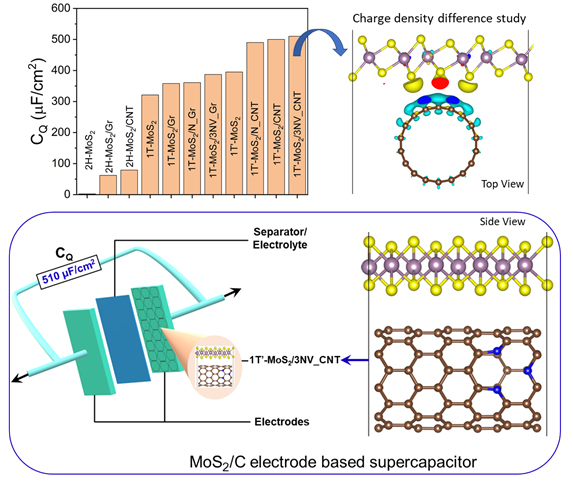 A research paper titled “First Principle Identification of 2D-MoS2 based Composite Electrodes for Efficient Supercapacitor Application” is published by Samadhan Kapse, PhD student, as First Author and Bennet Benny, BSc Physics Student, (Same Contributed First Author) in the Journal of energy storage, Elsevier having an Impact Factor of 6.583. The paper publication has been guided and supervised by Dr Pranab Mandal (Co-Author) and Prof Ranjit Thapa (Corresponding Author) from the Department of Physics, SRM University-AP.
A research paper titled “First Principle Identification of 2D-MoS2 based Composite Electrodes for Efficient Supercapacitor Application” is published by Samadhan Kapse, PhD student, as First Author and Bennet Benny, BSc Physics Student, (Same Contributed First Author) in the Journal of energy storage, Elsevier having an Impact Factor of 6.583. The paper publication has been guided and supervised by Dr Pranab Mandal (Co-Author) and Prof Ranjit Thapa (Corresponding Author) from the Department of Physics, SRM University-AP.
1T Molybdenum disulfide (1T-MoS2) has been widely studied experimentally as an electrode for supercapacitors due to its excellent electrical and electrochemical properties. Whereas the capacitance value in MoS2 is limited due to the lower density of electrons near the Fermi level, and unable to fulfil the demand of industry i.e. quantum capacitance preferably higher than 300 μF/cm2. Here, we investigated the performance of 2H, 1T, and 1T’ phases of MoS2 in its pristine form and heterostructures with carbon-based structures as an electrode in the supercapacitors using density functional theory. Specifically, we reported that the underneath carbon nanotube (CNT) is responsible for the structural phase transition from 1T to 1T’ phase of MoS2 monolayer in 1T’-MoS2/CNT heterostructure. This is the main reason for a large density of states near the Fermi level of 1T’-MoS2/CNT that exhibits high quantum capacitance (CQ) of 500 μF/cm2 at a potential of 0.6 V. Also, we observed that the nitrogen doping and defects in the underneath carbon surface amplify the CQ of heterostructure for a wider range of electrode potential. Therefore, the 1T’-MoS2 /N doped CNT can be explored as an electrode for next-generation supercapacitors.
Today’s increasing demand for energy storage technologies is highly dependent on batteries, fuel cells, supercapacitors, etc. The supercapacitors are greatly efficient due to advantages such as high power density, wide operating temperature range, large charge-discharge cycles. The recent focus of researchers is to find promising electrode materials for supercapacitor application. Among all reported works, the MoS2 nanosheet is found to be a prime candidate for supercapacitors with a high power density as well as energy density. Therefore, it is important to understand the origin of capacitance in MoS2 and their composites to design promising electrodes for supercapacitors. Also, the identification of ideal MoS2 based composites for efficient supercapacitor application is a grand challenge using only experimental approaches.
Using density functional theory, we can identify the promising electrode materials for supercapacitor application based on various graphene, 2D metal chalcogenides and their heterostructures. The quantum capacitance (CQ) is the cost-effective method to estimate the performance of any low density of states materials such as graphene, MoS2, etc towards supercapacitors.
- Published in Departmental News, News, Physics News, Research News
Eco-friendly and economic production of Ammonia
 SRM Univeristy-AP is proud to announce that Prof. Ranjit Thapa, Department of Physics, has obtained a prestigious SERB-DST grant of Rs. 32 lakhs for a period of three years for his project, “Design Principle of Single Atom Catalyst for Nitrogen Fixation over HER: Energy Parameter, Electronic Descriptor and Database”.
SRM Univeristy-AP is proud to announce that Prof. Ranjit Thapa, Department of Physics, has obtained a prestigious SERB-DST grant of Rs. 32 lakhs for a period of three years for his project, “Design Principle of Single Atom Catalyst for Nitrogen Fixation over HER: Energy Parameter, Electronic Descriptor and Database”.
Ammonia (NH3) is the prime source of fertilizers and an important carrier of energy too. Ammonia can be stored in its chemical form for a long and it is easy to transport. So now researchers are looking forward to using ammonia in place of hydrogen as an energy source. But the production of ammonia with existing techniques needs more energy compared to the energy it stored in its chemical bond. So, an alternative process that is environmentally friendly and cost-effective is needed to be in place.
In 2019 the global production capacity of ammonia is 235 million metric tons and will increase to 290 million metric tons by 2030. The importance of ammonia is due to its application in broad and diverse fields, such as fertilizers, textiles, pharmaceuticals, and is a carbon-free energy carrier. The Haber-Bosch process is used for the synthesis of ammonia (NH3) from N2 and H2 using Fe based catalyst. But the process emits carbon dioxide (CO2) (1.5 tons of CO2/tons of NH3 production) requires high pressure and temperature and consumes around 2% of the global supply of energy. Electrocatalytic N2 fixation (N2 + 6H+ + 6e− → 2NH3) showed great potential due to the possible use of atmospheric nitrogen and hydrogen derived from water through electrolysis and in mild conditions. However, the slow kinetics of N2 adsorption, splitting of the strong N≡N bond are the challenges for the electrocatalytic NRR process. In the electrocatalytic NRR process, the fast reaction kinetics of hydrogen evolution reaction is the greatest obstacle. To solve these challenges, the search for various types of catalysts is on the roll.
To date, trial and error methods have been used to synthesize the catalysts for the electrocatalytic NRR process. Thanks to the rapid development of density functional theory-based computational methods, the intermediate steps during NRR can be identified at the atomic level, the underlying principles can be understood, and a large space of catalysts can be checked for efficient NRR within a limited time. Without understanding the correct electronic structure of SAC and its correlation with the overpotential of NRR and defining the correct energy parameter to define “NRR over HER” and “N2 binding over H binding free energy”, we can never design the best catalyst cost-effectively. We will address these problems through this project’s objectives.
The project will help to design the best single-atom catalyst for the reduction of nitrogen (from the air) through the electrocatalytic process and convert it into ammonia. The designed catalyst can be synthesis by the industry and can be used for NRR.
This project will help a step forward towards more ammonia production for the uses in the agriculture sector, energy sector, and related sector.
- Published in Departmental News, News, Physics News
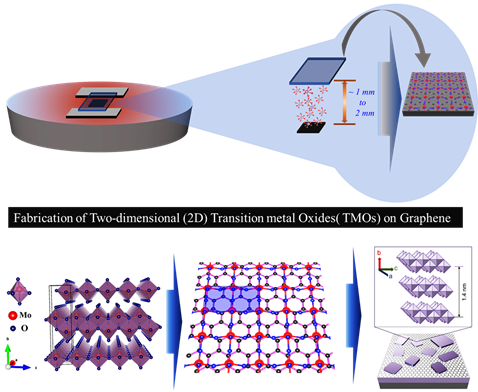
A novel method of synthesising 2D transition metal oxide layers
Patent application No: 202241005220
Publication date: 11/02/2022
Title: Two-Dimensional Transition Metal Oxide Layers and a method for their Synthesis
Inventors: Dr. Jatis Kumar Dash, Shaik Md. Abzal, Kurapati Kalyan, Sai Lakshmi Janga
Department of Physics, SRM-University-AP, Andhra Pradesh
The Department of Physics is glad to announce that Dr Jatis Kumar Dash and his PhD Scholars Shaik Md. Abzal, Kurapati Kalyan and Sai Lakshmi Janga have got their patent “TWO-DIMENSIONAL TRANSITION METAL OXIDE LAYERS AND A METHOD FOR THEIR SYNTHESIS” published on February 11, 2022.
About the Patent
Extensive use of portable electronic products and the rapidly growing commercial markets in smart electric appliances have created a seemingly high demand for flexible, wearable high-performance photoelectric devices and energy storage technology. In the search for new materials to meet these criteria, one promising solution may be the two-dimensional (2D) material heterostructures, assembled by stacking different conventional 2D materials (for example, graphene, transition metal oxides, carbides, and chalcogenides) in hetero-layered architectures.
These 2D materials stackings are ultrathin layered crystals that show unusual physicochemical properties at few-atom thickness. These 2D heterostructures offer several key advantages for the next-generation devices such as (i) atomically thin 2D nanosheets provide a larger surface area due to complete exposure of the surface atoms, (ii) the edge sites in 2D nanosheets are chemically more reactive than their basal planes and the open gaps enable the intercalation of electrolyte ions and (iii) the high mechanical strength and flexibility at atomic dimensions allow them to be used in the next-generation wearable electronics.
But the growth and stacking of 2D materials is always a challenge. Also, the existing growth tools are complex and expensive. Here, at SRM University-AP, we have fabricated the large-area ultra-thin 2D transition metal oxide (TMO) layers using an easy and cost-effective method. In addition, these 2D TMO layers are further integrated to different other 2D materials for their use in nano-electronic devices. Our work shows the great potential of ultra-thin TMOs in 2D-material-based flexible electronics.
Social Implication
2D materials are the prime candidates for making flexible, wearable, foldable and transparent self-powered smart electronic devices. The next-generation smart electronic devices will be made of 2D materials heterostructures which will need less operating power, less consumption of materials and will have ultimate scalability.
The team is also in the process of optimization and aims to make prototype flexible 2D supercapacitors, photodetectors, ultrathin transistors, and various sensors.
- Published in Departmental News, News, Physics News, Research News